Abstract
Background: Inflammatory bowel disease (IBD) is associated with a widespread breakdown of glycosaminoglycans, which are normally attached to mucin and help to form a protective barrier separating bacteria from the intestinal epithelium. N-acetylglucosamine (NAG) is a naturally occurring amino sugar precursor for epithelial glycosaminoglycan synthesis. We hypothesize that NAG administration can alleviate IBD-related inflammation by increasing glycosaminoglycan synthesis, which would result in more glycosaminoglycan attachments to the protective mucin layer. Objective: To assess the efficacy and safety of NAG as an adjunct therapy for IBD. Methods: Thirty-four adult IBD patients were recruited into a pragmatic open-label clinical trial. A daily dose of 6 g NAG was taken orally for 4 weeks. Self-reported IBD symptom scores were assessed at baseline and after 4 weeks of treatment. Results: Overall, 88.1% (30 out of 34) patients reported NAG helped with their IBD symptoms: 58.8% of patients reported improvement in abdominal pain with a 49% reduction in symptom score (P=0.00022); 64.7% of patients reported improvement in diarrhea with a 47% reduction in symptom score (P=0.0003). Significant reductions in symptom score were also observed for nausea, passage of mucus, and rectal bleeding (effect size=41%-55%, all P<0.05). The medication was well tolerated, and treatment response did not differ between genders. Conclusion: This pragmatic open-label clinical trial demonstrated that NAG could be an efficacious adjunctive treatment for IBD. Placebo-controlled studies with larger sample sizes should be considered in the future.
Introduction
Inflammatory bowel disease (IBD) affects approximately 1.4 million people in North America and 2.2 million people in Europe, with a peak onset age of 15 to 30 years.1 IBD mainly comprises 2 types of chronic intestinal disorder: Crohn’s disease (CD) and ulcerative colitis (UC). CD generally involves the ileum and colon but can also affect any region of the intestine, often discontinuously. UC involves the rectum and may affect parts of the colon or the entire colon in an uninterrupted pattern.2
Several classes of medications are currently used to treat IBD, including 5-aminosalicylic acids (eg, mesalamine [Pentasa]), corticosteroids (eg, budesonide [Entocort]), immunosuppressants (eg, methotrexate), and tumor necrosis factor–α (TNF-α) inhibitors.3,4 However, these medications often have limited to moderate clinical efficacy and potential serious side effects, particularly in the cases of corticosteroids and immunosuppressants. With current pharmacological treatments, 13% to 20% of CD patients have a chronic active course of disease activity, 67% to 73% have a chronic intermittent course, and only one-tenth have prolonged remission.5-7 After 20 years, most patients with CD require surgery.8 Similarly, in the case of UC, 18% of patients have a chronic active course of the disease, 57% experience intermittent relapses, and only 25% of patients are in prolonged remission with current pharmacological treatments.4 Ten years after diagnosis, about a quarter of UC patients will require colectomy.9 These statistics illustrate the modest clinical efficacy of the current treatments and demonstrate the need for new, more efficacious IBD treatments.
Mucin, a family of heavily glycosylated proteins, forms a protective barrier to separate bacteria from the intestinal epithelium.10 The outer loose mucin layer is the site of attachment of intestinal bacteria colonies, which play an important role in normal intestinal physiology.11,12 The inner mucin layer is normally impenetrable to bacteria and serves as a physical barrier separating the bacteria from the intestinal epithelium.13 In patients with IBD, the release of TNF-α increases the gene expression of matrix-degrading metalloproteases, which results in a widespread breakdown of glycosaminoglycans, including those attached to mucin.14-19 Genetic variants in the gene encoding for metalloproteases are strong risk factors for IBD.20 The degradation of glycosaminoglycans that are attached to mucin in IBD patients is selective toward the complex glycosaminoglycans. Larsson and colleagues showed that the glycosylation profile of mucin is altered in patients with IBD.21 The mucin layer in patients with active IBD is much thinner compared to healthy controls and has fewer complex glycosaminoglycans attaching to them.21 The magnitude of the complex glycosaminoglycans degradation is associated with the severity of IBD and is reversed upon IBD remission.21 As a result of the thinner mucin layer, bacteria can easily penetrate the normally impenetrable inner mucin layer and trigger inflammation, vascular leakage, and microthrombosis in patients with IBD.22
Despite the promising evidence, the efficacy of NAG on IBD has not been tested in a clinical trial setting with adult IBD patients. Here, we report the findings of a real-world pragmatic clinical trial testing the efficacy of NAG for the treatment of IBD.
N-acetylglucosamine (NAG) is a naturally occurring amino sugar and can be used as a precursor for epithelial glycosaminoglycan synthesis. Compared to other glycosaminoglycan precursors, such as glucosamine sulphate, NAG is 10 to 20 times more preferentially incorporated into the intestinal mucin layer due to deficiency in N-acetylating amino sugars in IBD patients.23 We hypothesized that NAG administration could alleviate IBD-related inflammation since increased glycosaminoglycan synthesis would result in more complex glycosaminoglycan attachments to the mucin layer and therefore a thicker mucin layer in theory. This hypothesis is supported by in vitro, in vivo, and clinical evidence. Human peritoneal mesothelial cells treated with NAG in vitro showed a dose-dependent increase in glycosaminoglycan synthesis.24 Experimental administration of NAG in vivo also significantly reduced indomethacin-induced intestinal inflammation (a model of IBD) in mice.25 Clinically, NAG adjunct therapy was able to dramatically improve the IBD symptoms in children with severe treatment-resistant IBD.26 Notably, 8 out of 12 children showed noticeable improvement in IBD symptoms. Out of the 7 children who suffered from symptomatic strictures at baseline, only 3 required surgery after a 2.5-year follow-up period. Tissue biopsy showed that NAG treatment resulted in clear histological improvements and thickening of the glycosaminoglycan layer.26 Despite this promising evidence, the efficacy of NAG on IBD has not been tested in a clinical trial setting with adult IBD patients. Here, we report the findings of a real-world pragmatic clinical trial testing the efficacy of NAG for the treatment of IBD.
Methods
Study Design and Participants
We conducted this pragmatic, open-label clinical trial in Toronto, Ontario, Canada. Thirty-four patients were recruited via their physicians. Participants were eligible if they were aged 18 years or older, had been diagnosed with IBD, were not fully satisfied with the current medication, wanted to try NAG to treat their IBD, and could provide consent. Participants who are allergic to shellfish were excluded from the study since shellfish is the source of NAG. Since NAG has no known drug interactions, no adjustments were made to any of the patients’ concurrent medications. The first recruitment was on May 17, 2013, and the last follow-up was on August 12, 2014. NAC was provided in powder form by Wellesley Therapeutics Inc (Toronto), but no other support was received from the company. All participants provided informed consent in accordance with the principles expressed in the Declaration of Helsinki.
Procedures
People who met the inclusion criteria and gave consent were asked to provide their demographic (age and gender) details and information about their IBD treatment history. An IBD symptom score questionnaire was administered by a pharmacist to measure the severity of these 6 IBD symptoms: abdominal pain, nausea, diarrhea, passage of mucus, rectal bleeding, and weight loss (score 0 to 10; where 0=absent; 10=symptoms as bad as it could be). A 4-week supply of NAG was sent to each participant with instructions on how to use it. NAG powder was supplied in a 2-g sachet (Villicote,® Wellesley Therapeutics Inc). The participants were instructed to reconstitute the NAG powder in water. A total daily dose of 6 g was taken orally in 3 divided doses of 2 g each. A pharmacist made the follow-up phone call with the participants after 4 weeks of NAG treatment and administered the IBD symptom score questionnaire again.
The primary outcome of this study was the improvement in IBD symptom scores after 4 weeks of NAG treatment (calculated as baseline symptom score minus follow-up symptom score). Participants were not paid for participation, nor did they receive additional NAG. Secondary outcomes assessed at 4 weeks were medication adherence (ie, asking the question “How many sachets are you using daily?”), overall efficacy (ie, asking the question “Overall, did the product help you with your symptoms?”), and the side effects profile.
Statistical Analyses
A sample size of 34 with repeated measure design conferred 80% power (with 2-side P=.05) to detect an absolute difference of 30% in all 6 IBD symptoms scores (assuming mean score=4 with standard deviation [SD]=2.5). Comparisons of IBD symptoms scores between baseline and follow-up were done by paired t-tests (Mann-Whitney tests gave similar results). The effect of gender on NAG efficacy was assessed by paired t-tests. Statistical analyses were performed using Stata 12 (StataCorp, College Station, Texas). Graphing was performed using R statistical programing.
Results
Of the 34 participants who were recruited, 19 were female (55.8%). The average age was 51.9 years (SD=16.8 y). The self-reported symptoms scores at baseline are presented in Table 1. Overall, patients reported higher baseline symptom scores for abdominal pain, diarrhea, and passage of mucus than nausea, rectal bleeding, and weight loss (Table 1).
Table 1. Baseline Demographics and Symptoms Scores
| Mean (Standard Deviation) |
N | 34 |
Age (y) | 51.9 (16.8) |
Gender, (% Women) | 55.8% |
Baseline Symptoms Scoresa | |
Abdominal pain | 4.3 (3.4) |
Nausea | 2.2 (2.8) |
Diarrhea | 4.5 (3.3) |
Passage of mucus | 3.5 (3.3) |
Rectal bleeding | 3.0 (3.2) |
Weight loss | 1.9 (3.0) |
Gas | 1.9 (3.2) |
aSymptom scores ranged from 0 to 10; where 0=absent; 10=symptoms as bad as it could be
Four weeks of NAG treatment significantly reduced the symptom scores for the most commonly reported IBD symptoms. In the case of abdominal pain, the average symptom score was reduced by 49% (Figure 1A; mean baseline score=4.26, mean follow-up score=2.18, P=.00022). Of the patients treated with NAG, 58.8% reported improvement in abdominal pain, whereas only 8.8% of the patients reported worsening of symptoms (Table 2). In the case of diarrhea, 64.7% of the patients reported improvement in diarrhea symptoms, with the average symptom score reduced by 47% (Figure 1B and Table 2; mean baseline score=4.53, mean follow-up score=2.38, P=0.0003). NAG treatment also reduced the average symptom score for passage of mucus by 43.5% (Figure 1C and Table 2; mean baseline score=3.47, mean follow-up score=1.82, P=0.0004).
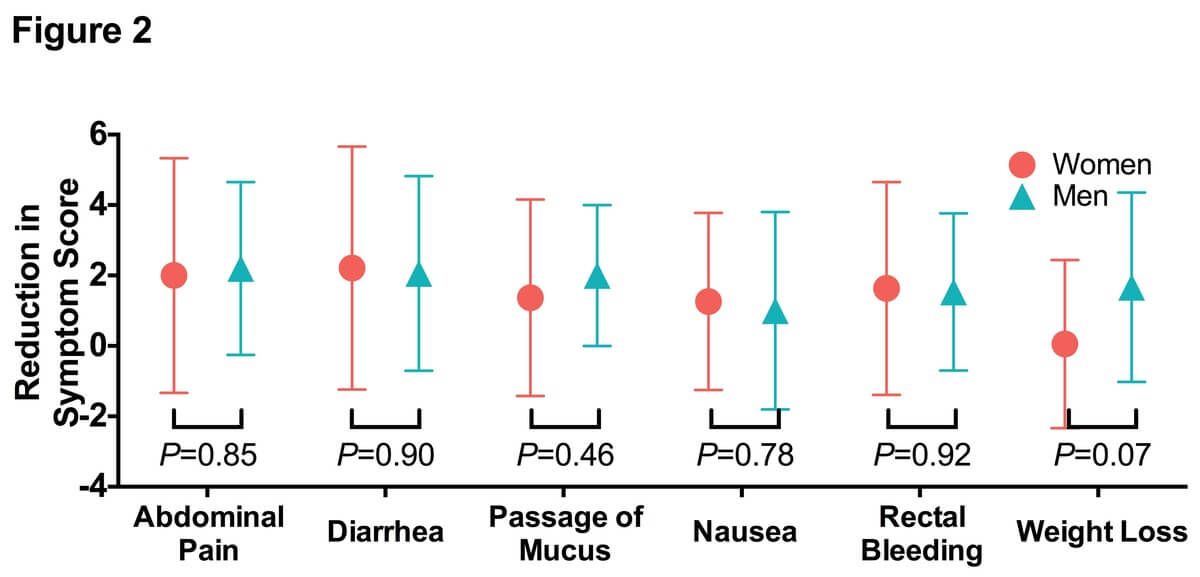
Similar trends were observed with the less commonly reported IBD symptoms. NAG treatment reduced the average nausea symptom score by 55% (Figure 1D; mean baseline score=2.2, mean follow-up score=1.05, P=0.015). In all, 41.2% of the patients reported improvement in nausea symptoms (Table 2). NAG treatment also significantly alleviated symptoms of rectal bleeding (Figure 1E; mean baseline score=3.0, mean follow-up score=1.44, P=0.001), with 52.9% of the patients reporting improvement in nausea symptoms (Table 2). We did not observe any significant change in weight loss (Figure 1F; P=0.10), possibly due to the short follow-up period. The efficacy of NAG on IBD symptoms was comparable between men and women (Figure 2).
Figure 1. Four weeks N-Acetylglucosamine treatment significantly alleviated inflammatory bowel disease symptoms. A) Each blue (man) or red (woman) symbol represents a single participant with the lines representing the trajectory of the symptom scores from baseline to follow-up. The black dots and error bar represented the group mean ± standard derivation. P-values were from paired t-tests. Symptom scores ranged from 0 to 10, where 0=absent; 10=symptoms as bad as it could be.
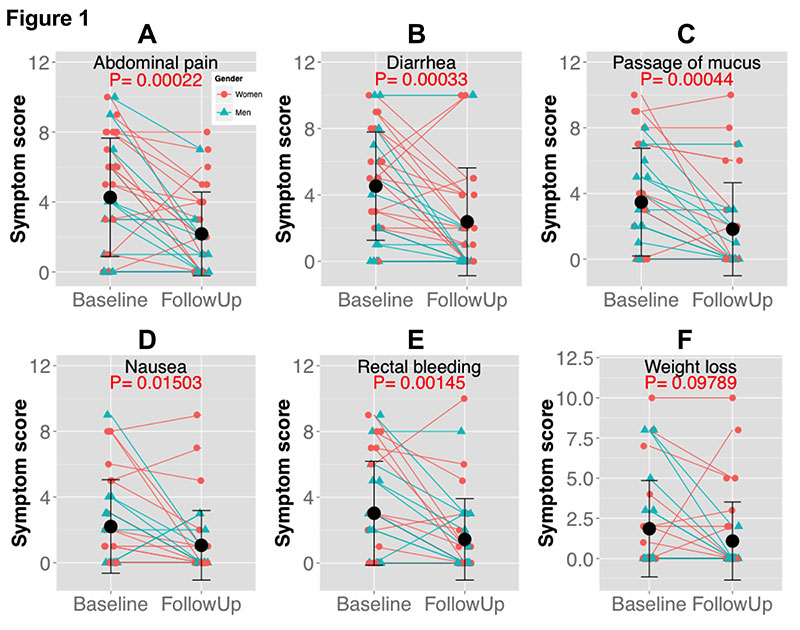
Figure 2. N-Acetylglucosamine treatment reduced inflammatory bowel disease symptoms with similar efficacy between genders.
We observed a very limited side effect profile associated with NAG treatment. The self-reported side effects were similar to the IBD symptoms. Less than 10% of patients reported worsening of symptoms after NAG treatment. The most notable side effect was an increase in nausea after NAG treatment reported by 3 patients (Table 2). Self-reported medication adherence was also high in this study, with 65% people reported that they are fully adherent to the 6 g per day treatment, and 30% people reported partial adherence (4 g/d). Most of the participants dissolved the medication in water, coffee, or juice. Overall, 88.1% (30 out of 34) patients reported that NAG helped with their IBD symptoms.
Table 2. Patients Reporting Symptom Improvement After Receiving N-Acetylglucosamine
Symptoms | N | Participants Reporting Symptom Improvement, % | Participants Reporting No Change in Symptoms, % | Participants Reporting Symptoms Worsening, % |
Abdominal pain | 34 | 58.8 | 32.4 | 8.8 |
Nausea | 34 | 41.2 | 47.1 | 11.8 |
Diarrhea | 34 | 64.7 | 26.5 | 8.8 |
Passage of mucus | 34 | 52.9 | 41.2 | 5.9 |
Rectal bleeding | 34 | 52.9 | 38.2 | 8.8 |
Weight loss | 34 | 29.4 | 61.8 | 8.8 |
Gas | 34 | 26.7 | 70.0 | 3.3 |
Discussion
In this pragmatic open-label clinical trial, we found that 4 weeks of NAG treatment significantly alleviated self-reported IBD symptoms, including abdominal pain, diarrhea, passage of mucus, nausea, and rectal bleeding. The medication was very well tolerated and treatment response did not differ between genders. Our findings extend previous animal and pediatric evidence and represent the first clinical evidence that NAG is an effective treatment, at least as an adjunct therapy, for IBD symptoms in adult patients.25,26 The proportion of patients who reported improvement in their IBD symptoms (30%-60%) in our study is similar to the 40% to 67% previously reported by Salvatore and colleagues in children with treatment-resistant IBD.26 Our results are in agreement with the findings of previous animal data that showed that oral supplementation of NAG treatment can significantly improve upper and lower small intestine inflammation and intestinal injury.25 Considered together, these data provide further evidence that glycosaminoglycans play an important role in normal gastrointestinal (GI) physiology and that preventing or reversing the degradation of these glycosaminoglycans in IBD patients could be a useful therapeutic direction for treatment.
Theoretically, there are 3 pathways through which NAG treatment could improve intestine inflammation and intestinal injury. The first pathway is through enhancing mucin glycosylation, resulting in better integrity of the GI epithelial barrier. The glycosylation pattern of mucin is dramatically altered in patients with IBD.21,27,28 Salvatore and colleagues observed that NAG treatment resulted in an enhancement of matrix and epithelial expression of mucin, as well as increased intracellular density of monomeric NAG residues in biopsy samples in children treated with NAG, suggesting that the clinical IBD response associated with NAG treatment is at least partially mediated by enhancing mucin glycosylation.26
The second possible pathway is through enhancing heparan production (a polysulfated polymer consisted of NAG and glucuronic acid), resulting in better epithelial restitution by binding growth factors such as basic fibroblast growth factor that rely on the presence of heparan sulfate proteoglycans. This is supported by the observation that unfractionated heparin (another highly polysulfated polymer consisting of NAG and glucuronic acid) administration has been shown to be beneficial in the treatment of IBD.29
A third mechanism may involve enhanced T cell‒receptor glycosylation. The N-glycosylation profile of T-cell receptors on lamina propria T lymphocytes is dysregulated in ulcerative colitis patients.30 NAG treatment could alleviate the N-glycosylation dysregulation on T-cell receptors in a mouse model of multiple sclerosis.31 Therefore, it is possible that NAG acts in a similar manner to enhance T cell‒receptor glycosylation in IBD patients. Currently, the relative contribution of these pathways to the clinical efficacy of NAG treatment has not been established and requires further investigation.
Toxicity
In this study, we did not observe any notable toxicity associated with NAG treatment. Most of the adverse effects reported by the patients were exaggeration of IBD symptoms (although less than 10% of patients reported worsening of symptoms in this study). Overall, no patients discontinued NAG treatment during the 4-week follow-up period, and the self-reported drug adherence was very high in this study. Our toxicity findings were highly consistent with previous NAG toxicity studies in human and in experimental animals reported in the literature.32-34 Furthermore, NAG is detected at high concentrations naturally in human milk and has been used as a human nutritional supplement in North America for many years without reported adverse effects.35 These data suggest that NAG treatment is safe and is unlikely to result in significant toxicity in IBD patients.
Limitations
Our data should be interpreted in the context of several limitations. First, we recognize that our study did not take pretreatment and posttreatment biopsy samples for histological assessment of IBD severity. However, the Salvatore et al study (2000), which had very similar dosing regimens, showed that NAG treatment resulted in noticeable histological improvement in the GI epithelium.26 Second, our study utilized an open-label pragmatic design that did not have a placebo treatment arm; thus, we were not able to assess the magnitude of placebo effect. Placebo-controlled studies are necessary to confirm our findings. However, some of the symptoms we assessed, such as passage of mucus and rectal bleeding, are objective, and the improvement we observed is unlikely simply a placebo effect. Third, our sample size limited our ability to assess the interaction between NAG with other concurrent IBD mediations (ie, Was the efficacy of NAG treatment different in patients treated with concurrent 5-aminosalicylic acids than patients treated with immunosuppressants?). Overall, despite these limitations, the high degree of consistency between our study and previous animal and human data strongly suggest that NAG could be an efficacious treatment for IBD. NAG may be a safe and inexpensive addition to current treatment regimens in IBD. Although we did not observe any heterogeneity among different types of IBD, it is reasonable to hypothesize that NAG treatment will work the best in the part of GI tract with the highest concentration of NAG. Future studies could test this hypothesis with the use of an NAG enema for the treatment of more distal disease.
Conclusion
In conclusion, this open-label, real world, pragmatic clinical trial demonstrated that NAG could be an efficacious adjunctive treatment for IBD. Placebo-controlled studies with larger sample sizes should be considered. Larger studies may also be used to determine which of the conventional medication work most effectively with NAG.









