Abstract
Poor blood circulation often manifests as small but chronic temperature differences in the peripheral extremities, and the surface of the skin may be an indication of abnormal blood flow and more serious vascular or circulation disorders. This study investigates the effect of Oligonol, a highly bioavailable source of low–molecular weight polyphenols extracted from lychee fruit, on peripheral blood circulation using skin thermography. The results suggest that Oligonol might act as a vasodilator and be an effective treatment for a variety of vasoconstriction symptoms such as cold hands and feet, shoulder discomfort, and diabetes-related vascular problems.
Introduction
Phenolic-rich foods and extracts have been shown to have antioxidant, antibacterial, antiinflammatory, antiallergic, hepatoprotective, antithrombotic, antiviral, anticarcinogenic, vasodilatory, and neuroprotective properties.1-4 Proanthocyanidins (also called flavanols) are a large class of polyphenols found in cocoa, grapes, persimmons, and bananas. Proanthocyanidins are structurally characterized as polymers of catechin and have a high molecular weight. While they have been shown to have strong in vitro antioxidant activity, their in vivo antioxidant activity is not as high as expected due to the poor bioavailability when taken orally.
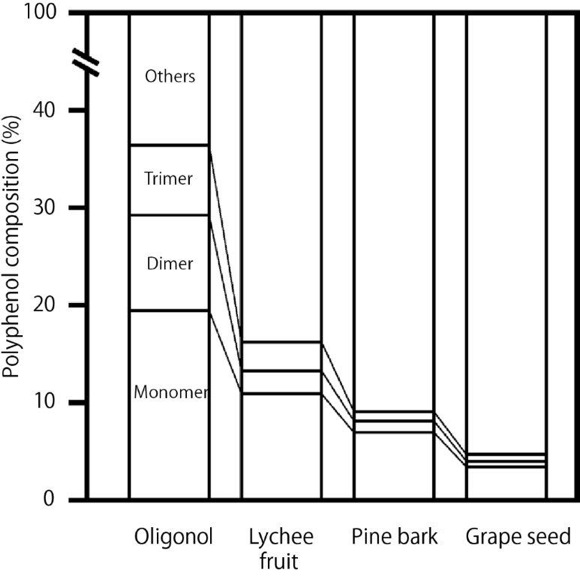
Lychee fruit (Litchi chinensis, Sapindaceae) has been consumed in China and Southeast Asia since ancient times. Lychee is rich in polyphenols; its polyphenol content per edible part has been reported to be second only to strawberries.5 Another particular feature of lychee is that it contains a comparatively large number of the less common A-type proanthocyanidin units.6 Oligonol is a flavanol-rich lychee extract processed to convert the high–molecular weight proanthocyanidins into low–molecular weight proanthocyanidins to improve bioavailability. The patented technology used to produce Oligonol has been previously described7 with some modifications involving mixing proanthocyanidins with tea extract instead of L-cysteine. It has been found that the low–molecular proanthocyanidin content of Oligonol is markedly higher than that of lychee fruit, grapeseed, and pine bark polyphenols, all of which are similarly rich in proanthocyanidins (Figure 1).8 In addition, the oral bioavailability of Oligonol in humans is reported to be 3 to 4 times higher than that of unprocessed lychee fruit polyphenols.9 The safety of Oligonol has been established.10-12 Oligonol received notification as a new dietary ingredient from the US Food and Drug Administration (FDA) in 2007 and received notification as generally recognized as safe from FDA in 2014. Oligonol has been the subject of a number of clinical trials and has been shown to exhibit numerous health benefits, including protection against oxidative stress,13 prevention and treatment of hyperuricemia,14 reduction in fatigue,15,16 and reduction of visceral fat.17 It has also been shown to inhibit inflammatory markers following exercise.18,19
Figure 1. Low–molecular proanthocyanidin content of Oligonol vs that of lychee fruit, grapeseed, and pine bark.
In the United States, millions of people suffer from vascular disorders. A myriad of chronic vascular diseases such as peripheral artery disease, varicose veins, and hypertension result from an impaired circulatory system that limits the flow of blood to the heart, hands, and feet. Epidemiological studies suggest that a high dietary intake of flavanols is associated with reduced risk of vascular disease. Clinical studies have also shown that the consumption of certain flavanol-rich foods (eg, cocoa, tea, red wine) has improved various vascular biomarkers including improved endothelial function, reduced platelet reactivity, and lowered blood pressure.20-22 Early signs of many vascular disorders are those associated with poor peripheral circulation such as changes in temperature in extremities and skin surface. The purpose of this study was to investigate the clinical effect of Oligonol supplementation on peripheral circulation using infrared thermography.
Materials and Methods
The study was approved by the Research Ethics Committee of the Tsukuba University of Technology, Ibaraki, Japan. This was a single-blind, placebo-controlled crossover trial to assess the effect of Oligonol on peripheral circulation compared to a placebo in healthy adults. The study was conducted from August 1, 2008, to January 19, 2009, at the Center for Integrative Medicine, Tsukuba University of Technology. The effect of Oligonol on peripheral circulation was measured as skin temperature changes by infrared thermography (Model no. JTG-4310S, Japan Electron Optics Laboratory, Japan).
Six healthy subjects (3 male, 3 female; 28-40 y; body mass index<25) were supplemented with 50 mg Oligonol (Amino Up Chemical Co, Ltd, Sapporo, Japan) and a placebo (malt extract, dextrin) alternatively on different days. Subjects fasted for 12 hours prior to coming to the laboratory and changed into clothing specially designed for skin thermography measurement. To acclimate themselves, subjects were asked to stay calm and rest for 60 minutes in the temperature-controlled room (room temperature 26°C±1°C, humidity 50%) prior to oral administration of the samples. Thermographic measurements of changes in skin temperature were completed immediately before supplementation and every 30 minutes after supplementation for up to 120 minutes. The regions of interest (ROIs) for the thermographic measurements were the neck, shoulder, and right palm. The paired t-test was used to determine skin temperature differences within subjects between time periods. Welch's t-test was used to compare skin temperature between groups. A level of P.05 was recognized as statistically significant. Values are presented as mean and standard error of the mean.
Results
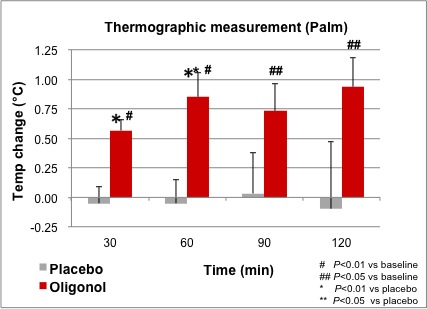
All 6 subjects completed the study. After 30 minutes following Oligonol ingestion, thermographic measurements showed a significant temperature increase in the right palm in the Oligonol group compared to the placebo group. Temperature changes between groups were significantly different at 30 minutes and 60 minutes. The temperature remained significantly above baseline levels for up to 120 minutes in the Oligonol group (Figure 2).
Figure 2. Comparison of thermographic measurement in right palm.
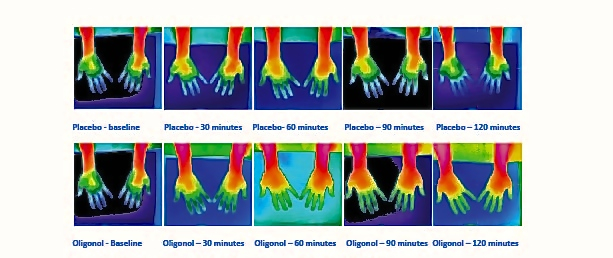
While thermographic measurements of the neck and shoulder trended toward an increase in temperature in the Oligonol group compared to placebo, there were no significant differences between groups. Within each treatment group, the temperature increased and remained significantly above the baseline temperature through 120 minutes (Figure 3). Figure 4 provides an example of thermographic imaging results of the palms of 1 subject in both groups.
Figure 3. Comparison of thermographic measurement in neck and shoulder.
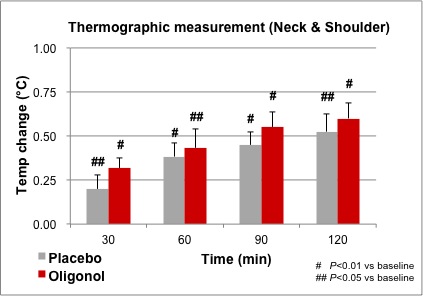
Figure 4. Thermographic images of palm of one subject, Oligonol vs placebo.
Discussion
This study was designed to evaluate the clinical effect of Oligonol on peripheral blood flow and was conducted by crossover design in which all subjects were assigned to both groups. The infrared skin thermography technique was utilized in the study to measure skin temperature as an established method.23 The results showed a significant elevation of body surface temperature in the before and after thermographs in the palms of all subjects in the Oligonol group, which suggests peripheral blood flow improvement in healthy people. Blood flow increases by polyphenols are suggested to be potential benefits on cardiovascular health and brain function.24 As Oligonol is a highly bioavailable source of low–molecular weight polyphenols of lychee fruit, it is suggested that Oligonol expresses its potential benefits at higher level than original lychee fruit polyphenol. Further studies are needed to confirm this conclusion.
Regarding the results of thermographic measurements of the neck and shoulder, there were no significant differences between groups. However, there was a significant increase in both groups compared to the baseline. Since the experiment was conducted after a 12-hour fast, the diet-induced thermogenesis (DIT) may have been caused by ingestion of the samples. DIT can be induced by a carbohydrate that was used in both sample capsules and occurs mainly in skeletal muscle; malt extract and dextrin were used in placebo capsules.25,26 DIT may have contributed to skin temperature increases on the neck and shoulder and raised the baseline measurement, and the temperature increases may have been caused by Oligonol ingestion. This may have resulted in the lack of significance observed in the temperature increases between groups in the proximal ROIs (neck and shoulder). The effect of the Oligonol, however, may have been more pronounced in the distal ROI (palm) because the baseline temperature of the palm may not have been affected by DIT compared to that of the neck and shoulder.
When blood endothelial cells are damaged by hyperglycemia or oxidative stress, production of nitric oxide (NO) and microcirculatory flow are reduced, resulting in vasoconstriction, inflammation, and clot formation. The underlying mechanism for Oligonol's effect on circulation may be a result of increased NO production. Previous studies have indicated that polyphenols might regulate NO production by the protein kinase C-dependent nicotinamide adenine dinucleotide phosphate-oxidase activation pathway.27 Moreover, a NO-dependent mechanism is further supported by the previous finding that Oligonol enhanced NO production by regulating phosphorylation and dephosphorylation of endothelial NO synthase (eNOS).28 The effect of Oligonol on NO production was investigated in bradykinin-stimulated vascular endothelial cells under high-glucose conditions. Oligonol prevented the impairment of eNOS activity induced by high glucose through reversing altered eNOS phosphorylation status. Briefly, when endothelial cells were stimulated by bradykinin (30 nm) and cultured in a medium with a high concentration of glucose, NO production was decreased. However, the reduction was recovered by treatment with Oligonol.27 We hypothesize that the elevation in temperature is a result of the increase of the blood through the vascular smooth muscle, resulting from polyphenol-enhanced NO production in the vascular endothelium. This mechanism may underlie the beneficial vascular health effects of Oligonol. Further studies with increased sample size are warranted.
Conflict of Interest Statement
Kentaro Kitadate and Kohei Homma are employed at Amino Up Chemical Co., Ltd. as research scientists. Amino Up Chemical is the developer and manufacturer of the Oligonol product used in this trial. The authors have not received personal financial gain from sales of the Oligonol product. All findings and views expressed in this paper are those of the authors and do not necessarily reflect the views of Amino Up Chemical.
Kazumasa Aoyagi, MD, PhD, does not have any conflict of interest and has not received any financial gain from sales of this product.








