Osteoporosis is estimated to affect more than 10 million Americans, with postmenopausal women at particular risk. Osteoporotic fractures can lead to postural changes, emotional distress, and chronic pain. Currently all medications approved by the US Food and Drug Administration for the treatment of osteoporosis carry slight to modest risks depending on the individual, the duration, the dosage, and the drug being used. Estrogen replacement therapy is not currently indicated for the treatment of osteoporosis, but it is approved for osteoporosis prevention. Interest in bioidentical hormone replacement as an alternative to conventional hormone replacement has increased in the last 12 years, although not always for logical or scientific reasons. The purpose of this review is to bring clinicians up to date on current information on the efficacy and safety of bioidentical hormones for the prevention of postmenopausal osteoporosis.
Abstract
Osteoporosis is estimated to affect more than 10 million Americans, with postmenopausal women at particular risk. Osteoporotic fractures can lead to postural changes, emotional distress, and chronic pain. Currently all medications approved by the US Food and Drug Administration for the treatment of osteoporosis carry slight to modest risks depending on the individual, the duration, the dosage, and the drug being used. Estrogen replacement therapy is not currently indicated for the treatment of osteoporosis, but it is approved for osteoporosis prevention. Interest in bioidentical hormone replacement as an alternative to conventional hormone replacement has increased in the last 12 years, although not always for logical or scientific reasons. The purpose of this review is to bring clinicians up to date on current information on the efficacy and safety of bioidentical hormones for the prevention of postmenopausal osteoporosis.
Introduction
Osteoporosis—characterized by low bone mineral density (BMD), compromised bone strength, and increased fracture risk— is the most common bone disease in humans.1 The National Osteoporosis Foundation estimates that more than 10 million Americans have osteoporosis and an additional 33.6 million have osteopenia.1 Together, osteoporosis and osteopenia affect the majority of postmenopausal women in the United States,2 and 1 in 2 white women can expect to experience an osteoporotic fracture at some time in her life.3 Osteoporosis and its related fractures increase with age, and annual hip fractures are expected to rise to 289,000 by the year 2030 in the United States.4 Osteoporotic fractures can have a profound effect on quality of life, leading to postural changes, anxiety, depression, and chronic pain.1 In addition, hip fractures are associated with increased risk of death within 1 year.5 The economic impact of osteoporotic fractures was estimated at $17 billion in 2005 and is expected to double or triple by 2040.1,6
The most recent guidelines from the American Association of Clinical Endocrinologists7 for the treatment of osteoporosis were published in 2010 (Table 1). Bisphosphonate medications are first-line agents for osteoporosis, but prescriptions of bisphosphonates decreased by more than 50% between 2008 and 2012 as awareness of rare but serious adverse effects emerged.8 These rare adverse effects include osteonecrosis of the jaw, atrial fibrillation, esophageal cancer, and atypical femur fractures.9 More common side effects that lead women to discontinue bisphosphonate medications include heartburn, headache, constipation, diarrhea, and joint pain. Raloxifene, a selective estrogen receptor modulator, is a second-line agent. Raloxifene is less effective than bisphosphonates, may increase hot flashes or night sweats, and is associated with a small increase in thromboembolism and stroke.10
aSource: The American Association of Clinical Endocrinologists.7
Because there is no ideal treatment for osteoporosis, prevention is extremely important. Lifestyle changes are foundational to the prevention of osteoporosis (Table 2), but some women, despite a healthy diet and lifestyle, continue to experience a decline in BMD after menopause. Several medications approved for the treatment of osteoporosis are also indicated for prevention (eg, alendronate, risedronate, zoledronic acid, ibandronate, and raloxifene). In addition, estrogen therapy is approved for the prevention of osteoporosis. Estrogen therapy’s protective effect against bone loss was one of the findings of the Women’s Health Initiative (WHI) first reported in 2002.11 The WHI, a randomized placebo-controlled trial of more than 27,000 postmenopausal women across the United States, established that treatment with conjugated equine estrogens (CEE) with or without medroxyprogesterone (MPA) decreased hip fracture risk by 33%. This benefit, however, was accompanied by a slight but statistically significant increased risk of thromboembolic disease and an even more slight but still statistically significant risk of invasive breast cancer.12
Since publication of data from the WHI, patients and providers have sought alternatives to conventional hormone replacement therapy (HRT). Among these alternatives is bioidentical hormone replacement therapy (BHRT). Although the US Food and Drug Administration (FDA) does not recognize a definition for “bioidentical hormone,” professional organizations have established that the term refers to a compound that structurally mimics an endogenous hormone.13 Bioidentical hormones include estrone, 17β-estradiol, estriol, progesterone, testosterone, and dehydroepiandrosterone (DHEA). Although compounded bioidentical formulations are not FDA-approved, it is important to note that there are many FDA-approved bioidentical hormone products on the market (Table 3). Comprehensive reviews of BHRT have been published elsewhere.14-17 The purpose of this review is to summarize recent data on the efficacy and safety of different formulations, delivery systems, and dosages of bioidentical hormones for the prevention of postmenopausal osteoporosis.
Effects of Bioidentical Hormones on Bone
Estradiol
Estradiol (17β-estradiol) is the most physiologically active form of estrogen and is endogenously produced at the highest level before menopause. Estradiol is available in a variety of FDA-approved formulations and in compounded formulas, including estradiol alone or in combination with other hormones. A PubMed search of human female clinical trials within the last 10 years with search terms of “estradiol” or “17β-estradiol” and “osteoporosis” or “bone” combined with a literature search of references from review articles produced 13 relevant studies on the effect of 17β-estradiol on BMD (Table 4). In this section, we summarize the data on different delivery systems of estradiol, clarify which are FDA-approved for osteoporosis prevention, address the efficacy of compounded formulations, and discuss duration of effect.
Although the studies on oral 17β-estradiol do not rival the WHI in size or duration, substantial data exist on its ability to maintain and increase BMD. Prevention of osteoporosis is an FDA-approved indication for its use, and the FDA asserts that the effects of oral estradiol are equivalent to the effects of CEE when given in equivalent doses. Oral estradiol from a conventional pharmacy comes in 3 standard dosages: 2 mg, 1 mg, and 0.5 mg. All of these have demonstrated efficacy at increasing BMD with 3 to 5 years of follow-up in placebo-controlled trials.18,19 Even studies that show a dose-dependent effect of estradiol on BMD demonstrate that the lowest oral dose (0.25 mg/d) is effective at preventing BMD loss in postmenopausal women.20-22
It has been suggested that transdermal application of 17β-estradiol might offer effective bone-sparing benefit with less risk than oral estrogens, especially when it comes to deep vein thromboses, ischemic strokes, triglyceride levels, and inflammatory markers.23 Like oral estradiol, transdermal estradiol patches are FDA-approved for osteoporosis prevention. The Kronos Early Estrogen Prevention Study (KEEPS), a randomized placebo-controlled study published in 2013, demonstrated comparable changes in BMD between women treated with oral CEE (Premarin, 0.45 mg/d) and those treated with transdermal 17β-estradiol patch (Climara, 50 μg/d) plus pulsed micronized progesterone with 4 years of follow-up.24 These results were consistent with earlier studies demonstrating bone-building efficacy of standard-dose (45 μg/d or more) transdermal 17β-estradiol patches.25-27 Evidence suggests that even an ultra low–dose (14 μg/d) transdermal 17β-estradiol patch can increase BMD28 and that this dosage has similar efficacy as raloxifene.29
Other forms of transdermal estradiol (eg, gel, emulsion, or spray) do not carry an FDA-approved indication for osteoporosis prevention, so their prescription for this purpose is considered an off-label use. Studies do suggest, however, that these delivery systems are effective ways to achieve therapeutic levels of estrogen that can positively affect BMD. Studies have shown that 17β-estradiol transdermal gel (Divigel, 1.0 mg/d) is comparable to oral estradiol for the maintenance of BMD and reduction of bone turnover and is equally effective as the estradiol patch (Estraderm TTS, 50 µg/d) at preserving BMD.30,31 It has also been shown that estradiol transdermal gel (1.0 mg/d) is effective at increasing BMD equally well in smokers and nonsmokers.32 A study to compare the efficacy of different doses of estradiol transdermal gel (delivering 0.75 g, 1.5 mg, or 3.0 mg 17β-estradiol /d) demonstrated significant increase in bone mass in the groups receiving 1.5 mg/d and 3.0 mg/d and that even the lowest dose prevented bone loss in postmenopausal women after 12 months of treatment.33 Newer delivery systems for transdermal estradiol include estradiol topical emulsion (Estrasorb) and estradiol transdermal spray (Evamist). Although no specific studies on osteoporosis were identified for these products, pharmacokinetic studies demonstrate that they also deliver therapeutic levels of estradiol.34,35
A variation on transdermal application of estradiol is transvaginal application. Like the gels, emulsion, and spray, transvaginal estradiol is also not indicated for the prevention of osteoporosis, so its prescription for this purpose would be considered an off-label use. There are minimal data to suggest that this delivery method might have a systemic effect on BMD. Two small studies demonstrated that transvaginal had a dose-dependent effect on BMD, with even the lowest dose (7.5 μg/d) preventing bone loss.36,37
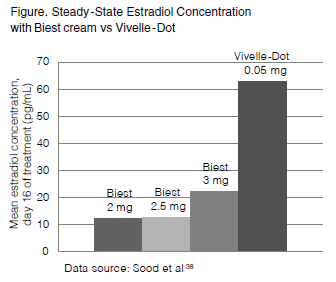
Despite the body of evidence demonstrating a bone-sparing effect of most commercial preparations of estradiol, questions remain about the reliability of compounded formulas to deliver an effective dose. There were no pharmacokinetic studies on compounded BHRT until a 2013 study published by Sood et al.38 This study compared serum hormone levels in women prescribed compounded estrogen cream (Biest 2.0 mg, 2.5 g, or 3.0 mg) plus compounded oral progesterone (100 mg) to those in women prescribed a conventional estradiol patch (Vivelle-Dot, 0.05 mg) plus oral micronized progesterone (Prometrium, 100 mg). The dosages were chosen because of their assumed bioequivalence in practice: most pharmacists consider Biest 2.5 g to be approximately equivalent to a standard 0.05-mg transdermal patch. Results showed, however, that Biest 2.5 mg yielded significantly lower steady-state levels of estradiol than the FDA-approved patch (area under the curve for estradiol=286 vs 917; P<0.001). Even the higher dose of Biest (3.0 mg) yielded lower steady-state levels than Vivelle-Dot (Figure). The authors of this study raised the question of whether transdermal compounded creams, at commonly prescribed doses, are sufficient to provide bone-protective effects.
Another consideration in regard to the efficacy of 17β-estradiol for the prevention of osteoporosis is its duration of effect. It is generally accepted that the bone-sparing effect of any estrogen therapy persists while taking the medication and then quickly dissipates within 3 to 4 years after discontinuing use. Data from the cumulative follow-up period of the WHI showed that the risk reduction in hip fractures observed during intervention did persist over the cumulative 13 years of follow-up in the CEE plus MPA group only (hazard ratio [HR]: 0.81 [95% confidence interval (CI): 0.68-0.97]),12 but the risk reduction attenuated in the CEE-only group, resulting in a cumulative incidence of hip fracture that was the same as placebo at 10.7 years of follow-up.39 This finding was consistent with other studies that have shown the incidence rate of fracture returns to that of women who have never taken HRT within 1 to 5 years of discontinuing use.40,41 Further studies are needed to determine whether the bone-sparing effect of 17β-estradiol persists or declines after discontinuing treatment.
Estriol
Estriol is the weakest of the estrogens, with approximately one-tenth the potency of estradiol. Estriol is not FDA-approved and therefore only available as compounded BHRT. It is the primary estrogen in both Biest and Triest, popular compounded formulas because of the perceived safety of estriol. Unfortunately, studies assessing the bone-sparing effect of estriol have included small sample sizes and yielded contradictory results. Several small Japanese studies, with sample sizes ranging from 24 to 151, have demonstrated that oral estriol (2 mg/d) increases BMD from 1.79% to 3.3% within 1 to 2 years of treatment.42-45 Two of these studies demonstrated comparable efficacy between CEE (0.625 mg/d) and oral estriol.42,43 Other studies, however, demonstrated no increase in BMD in postmenopausal women receiving oral estriol for 2 years.46-48 The contradictory results of these small studies provide insufficient data to draw any conclusions about the efficacy of estriol for the maintenance of BMD or reduction in fracture risk in postmenopausal women.
Estrone
Estrone increases endogenously after menopause when the adrenal glands play more of a role in hormone synthesis. Estrone and estradiol can interconvert reversibly, which suggests that these 2 hormones might have similar effects. Estrone is available in FDA-approved formulations, such as piperizine estrone sulfate (PES), or compounded formulations. The studies of estrone’s effect on BMD are minimal, with small-scale studies suggesting that PES can increase BMD in postmenopausal women with 2 years of follow-up.49,50 Because the available data on the bone-sparing effects of estrone are limited, however, it is impossible to conclude whether a prescription of estrone offers any benefit beyond that of estradiol.
Progesterone
Bioidentical United States Pharmacopeia–approved progesterone is available in over-the-counter creams, compounded hormone formulations, and 3 FDA-approved formulations. Progesterone is most commonly prescribed in combination with estrogen therapy—for women with intact uteri—to prevent the uterine hyperplasia that can result from unopposed estrogen. Only 100 mg daily or 200 mg for 12 days per month has been researched and shown to prevent estrogen-induced endometrial hyperplasia.51
It has been suggested by some that progesterone might play a direct role in bone health.52 Evidence to support this claim includes the following observations: progesterone appears to have a stimulating effect on osteoblasts in vitro, bone loss can occur during perimenopause when estrogen levels remain high and progesterone declines, and higher endogenous progesterone levels associated with ovulation correlate with improvement in markers of bone turnover.52 Clinical trials to support any beneficial effect of progesterone on BMD or fracture risk, however, are lacking. The minimal studies conducted to evaluate the effect of progesterone on BMD have yielded negative results.51,53,54 Evidence does not, therefore, support the use of progesterone alone to maintain BMD.
Testosterone
Testosterone is produced in small quantities by the ovaries and adrenal glands in women. Testosterone is not FDA-approved for the prevention of osteoporosis. There is some evidence, however, to suggest that testosterone plays a direct role in bone health: in vitro studies demonstrate that testosterone stimulates osteoblast differentiation and inhibits osteoblast apoptosis; in addition, orchiectomy in men produces rapid bone loss.55 In women, because testosterone can metabolize to estradiol, it is unclear which of these hormones has the most influence on bone density. Clinical trials on the effect of testosterone on BMD in women are limited. A recent study comparing the effect of implanted 17β-estradiol (50 mg) plus testosterone (40 mg) to no treatment showed an increase in BMD at 1 year in the treatment group that was not statistically significant.56 In a prospective trial of 34 postmenopausal women randomized to receive either estradiol implants (50 mg) or estradiol (50 mg) plus testosterone (50 mg) implants for 3 years, BMD increased more rapidly in the combined treatment group.57
DHEA
Dehydroepiandrosterone-sulfate (DHEA-S) and DHEA, precursor hormones that convert into testosterone and estrogens, decline with age. DHEA is not FDA-approved for the prevention of osteoporosis but is available as an over-the-counter supplement. DHEA can inhibit osteoclastic bone resorption,58 and low levels correlate with bone loss. A study of more than 1000 postmenopausal women demonstrated that high DHEA-S levels at baseline were associated with less bone loss, but the effect diminished over time.59 In a study of 208 healthy men and women (age range, 60-79 y), 12 months of supplementation with DHEA (50 mg/d) improved bone turnover specifically in women >70 years old.60 A smaller study, in contrast, demonstrated that 6 months of supplementation with DHEA (100 mg/d) produced no change in BMD.61 The DHEA and Well-Ness (DAWN) Study demonstrated that 12 months of supplementation with DHEA (100 mg/d) had a beneficial effect on BMD in women but not in men.62 Evidence suggests, though inconclusively, that DHEA supplementation may have some skeletal benefit in women.
Safety of Bioidentical Hormones
Risk assessment for any form of HRT is complex and depends on numerous factors, including age of the patient initiating treatment, duration of treatment, and biochemical individuality. Studies on the use of testosterone in women are limited, range from 1 month to 2 years, and raise safety concerns that include endometrial cancer, breast cancer, and cardiovascular disease.63 Safety data on the use of DHEA in women are even scarcer, providing insufficient evidence on its effect on the breast or the uterus and mixed evidence on its effect on the cardiovascular system.64 There is minimal research to distinguish the safety of bioidentical progesterone from synthetic progestins and even less to distinguish the safety of bioidentical estradiol from nonbioidentical estrogens. In the absence of comparable data, the FDA has asserted that the risks and benefits of all hormones at equivalent dosages should be assumed to be the same. According to the results of the WHI, this means that some of the most serious risks of any estrogen replacement (with or without progestin or progesterone) include thromboembolic events or invasive breast cancer.12
It has been suggested that thromboembolic risk associated with estrogen therapy might vary depending on the route of administration.23 First-pass hepatic metabolism of oral estrogens stimulates hepatic protein synthesis of inflammatory compounds, including c-reactive protein, insulin-like growth factor, and clotting factors—factors associated with increased thromboembolic risk. Data from a recent 3-year study in 75 women given transdermal compounded BHRT (Biest, progesterone, testosterone, and/or DHEA) support this assertion.65 Compounded transdermal BHRT produced no net thrombotic potential and favorable changes in inflammatory and immune markers. The Million Women Study, in which more than 1 million women were followed for a mean of 3.1 years, demonstrated that current use of oral but not transdermal estrogen therapy increased the risk of venous thromboembolism (relative risk: 1.42 [95% CI: 1.22-1.66] vs 0.82 [95% CI: 0.64-1.06]).66 These results supported previous findings of the Estrogen and Thromboembolism Risk (ESTHER) Study, a multicenter case-control study that demonstrated an increased risk of venous thromboembolism with oral but not transdermal use of estrogen and a 4-fold increased risk of myocardial infarction (MI) associated with oral estrogen compared to transdermal estradiol therapy.67 The ESTHER study also demonstrated that micronized progesterone had no thrombogenic effect, whereas norpregnane derivatives resulted in an almost 4-fold increase in thrombogenic events. In contrast to these data, a large population-based study conducted in the United Kingdom demonstrated comparable risk of MI for users of both oral and transdermal estrogens.68 In the United States, the KEEPS Trial compared the effect of oral CEE (Premarin, 0.45 mg/d), transdermal 17β-estradiol (Climara patch, 50 μg/d), or placebo on the progression of atherosclerosis in recently postmenopausal women.69 Participants in the 2 active treatment groups were also given micronized progesterone (Prometrium 200 mg 12 d/mo). The KEEPS Trial demonstrated equal rates of atherosclerotic progression at 4 years of follow-up in all groups and no statistically significant differences in the rates of MI, transient ischemic attack, stroke, or venous thromboembolic disease; those receiving 17β-estradiol also experienced improved glucose and insulin sensitivity with neutral effects on other biomarkers such as cholesterol and triglyceride levels.
The most compelling research comparing breast cancer risk of BHRT to that of conventional HRT suggests that the addition of bioidentical progesterone to estrogen therapy incurs less risk than the addition of a synthetic progestin. A recent case-control study conducted in France in 1555 menopausal women demonstrated a significant increase in breast cancer risk in groups receiving estrogen plus a synthetic progestin but no increased risk in those receiving estrogen plus micronized progesterone (odds ratio: 0.69-0.80, depending on duration of use).70 Similar results were reported in the French E3N cohort study (N=80,377), which was conducted for a mean follow-up of 8.1 years in postmenopausal women.71 Compared to no HRT, estrogen alone and estrogen plus a synthetic progestin (dydrogesterone or progestogen) yielded significantly increased risk for breast cancer, whereas the relative risk for estrogen plus natural progesterone was 1.00 (95% CI: 0.82-1.22). Consistent with these findings, a prospective, randomized trial of 77 women demonstrated that women in the group assigned CEE plus MPA experienced a marked increase in breast cell proliferation, whereas the group assigned to transdermal estradiol (1.5 mg/d) plus oral micronized progesterone (200 mg/d) did not.72 Results of the KEEPS Trial showed no statistically significant differences in the rates of breast cancer after 4 years of CEE, transdermal 17β-estradiol, or placebo.69 Although the KEEPS Trial was not designed to assess breast cancer risk and was too short to conclusively assess cancer risk, these findings punctuate those of the French studies that suggest bioidentical progesterone incurs less breast cancer risk than synthetic progestins when combined with estrogen therapy.
Conclusion
Randomized, double-blind, placebo-controlled trials on BHRT are lacking, but observational and case-control studies suggest that bioidentical estradiol (particularly oral or patch forms) is comparable to CEE for slowing bone loss, slightly increasing BMD, and reducing fracture risk. Minimal data suggest that DHEA and testosterone also have a beneficial effect on bone health in women. Transdermal administration of 17β-estradiol appears to carry less risk of thromboembolic events than orally administered CEE, and bioidentical progesterone might carry less risk of thromboembolism and breast cancer than synthetic progestins. Otherwise, it is most prudent to assume that BHRT carries the same risk profile as conventional HRT.
There is no ideal medication for either the treatment or prevention of postmenopausal osteoporosis. The choice of a prescription medication requires an astute understanding of the benefits and risks of each drug, while also understanding the risks of having osteoporosis as a woman ages. For postmenopausal women who experience bone loss despite a healthy lifestyle, BHRT might offer a viable alternative to other medications for the prevention of osteoporosis.
This article was a part of the August 2014 special Natural Medicine Journal
issue on endocrinology. To see the rest of the special issue, click here.