Abstract
Hormone replacement therapy (HRT) can benefit those with clear symptoms of hormone deficiency, especially older men who are looking for improved sexual function, muscle mass, mood, or cognition. Testosterone replacement therapy (TRT), when used along with appropriate lifestyle changes, can enhance a person’s quality of life. This review discusses the diagnosis of hormone deficiency and the potential benefits of TRT.
Introduction
The use of HRT has been on the rise in this country as Americans seek improved health and longevity. According to the Grand View Research group based out of San Francisco, the HRT market size in 2012 was estimated at 15.1 billion people. This estimate is based on pharmaceutical sales of hormones and is expected to increase at an annual rate of 8.2%.1
Both men and women seek advice about hormone replacement from healthcare professionals every day. When I was in practice years ago, almost all my patients looking to receive HRT were menopausal women. More recently, especially in the past 10 years, I have noticed an increase in men who are seeking medical advice for HRT. Unlike menopause, which generally occurs in a predictable time period, changes in male reproductive hormones usually occur gradually, throughout life.2 It is estimated that after the age of 40, men experience up to a 3% decrease in circulating total testosterone every year. One longitudinal study reported that low testosterone levels occur in 20% of men over the age of 60.3
According to some sources, prescriptions for TRT have as much as tripled in the past 10 years, but not without controversy.4 The US Food and Drug Administration (FDA) started reviewing TRT after a 2013 study published in JAMA reported a 30% increase in the risk of heart attacks and stroke in men using TRT.5 Since that time the relationship between testosterone therapy and heart disease or stroke has been unclear. In addition, the method of delivery (eg, injections, gels, creams) can affect the risk but even this research is mixed.
This article focuses on the clinically relevant aspects of TRT, including the diagnosis of hypogonadism (reduction or absence of testosterone secretion of the testes), overview of the testosterone hormone, the benefits and risks of TRT, the use of aromatase inhibiters and 5α-reductase (5-AR) inhibitors, contraindications for testosterone use, and methods of testosterone delivery. First, let’s start with a review of hypogonadism.
Hypogonadism
As men age, male hormones called androgens decrease and may lead to a condition known as hypogonadism. The most prominent androgen in men is testosterone, produced primarily by the testes. There are 2 classifications of hypogonadism: primary and secondary. Primary hypogonadism is also known as primary testicular failure. Causes include congenital abnormalities (eg, Klinefelter syndrome), mumps orchitis, hemochromatosis, testicular injury, and normal aging.
Secondary hypogonadism involves the decrease in testosterone production caused by conditions affecting the hypothalamus or pituitary gland. Secondary hypogonadism also includes reduction of testosterone caused by medications and obesity.6 It is very important that healthcare providers find the cause of low testosterone before initiating any therapy.
Testosterone Synthesis
Testosterone is synthesized from cholesterol and through conversion from progesterone. The production of testosterone is stimulated via luteinizing hormone (LH) in the pituitary gland, and most testosterone is secreted into the bloodstream by the testes.7 Bioactive metabolites of testosterone include dihydrotestosterone (DHT) and estradiol.8 Luteinizing hormone initiates the production of pregnenolone, which is converted to dehydroepiandrostone (DHEA), which is then rapidly converted to testosterone. Testosterone is the main circulating androgen and is converted to dihydrotestosterone (DHT) via 5-AR and estradiol via aromatase9 (Figure 1). Aromatase inhibitors block this conversion to estradiol, and 5-AR inhibitors block the conversion to DHT. Testosterone and DHT activate androgen receptors (ARs) in target cells, and the actions of estradiol are mediated by estrogen receptors (ERs).10 Androgen receptors are located in various places throughout the body including the prostate gland, adrenal gland, skeletal muscle, liver, and the central nervous system. Testosterone also has a weak bonding ability to estrogen and progesterone receptors, where DHT has a specific affinity to AR.11
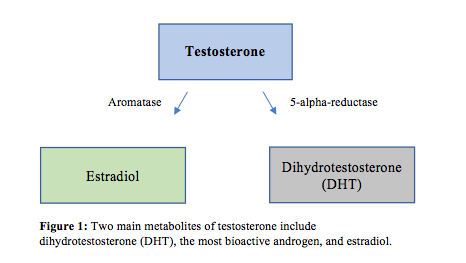
Testosterone Metabolites and Actions
Dihydrotestosterone (DHT) is the most bioactive androgen in men and is 2 times more potent than testosterone. Its superior potency is thought to be a consequence of DHT’s ability to bind more selectively to ARs compared to testosterone, including ARs in the prostate gland.11 Dihydrotestosterone has a larger impact on proliferation of prostate cells. 5-AR enzyme inhibitors such as finasteride have been shown to reduce prostate size in benign prostatic hypertrophy (BPH), evidence that corroborates DHT’s strong proliferative effect. However, this medication also increases serum levels of testosterone due to the inhibition of the conversion of testosterone to DHT.12 Both testosterone and DHT bind to sex hormone–binding globulin (SHBG) and albumin.13 Levels of SHBG increase with age, which may contribute to less bioavailable testosterone in older men.14 Bioavailable testosterone is free testosterone plus the testosterone that is carried in the blood stream via albumin. Total testosterone includes free testosterone and the testosterone carried by both albumin and SHBG. Elevated blood levels of DHT have not been shown to be any more clinically significant than levels of testosterone itself. There is no evidence that higher levels of DHT increase the risk of prostate cancer; the authors of a 2017 article published in Endocrine Reviews concluded that elevated levels of DHT in men receiving TRT is of no clinical concern.15 However, a study of 52 men with male androgenic alopecia (MAGA) found men with MAGA had higher levels of serum DHT, so perhaps running DHT lab tests on men with MAGA following TRT treatment might be helpful.16
According to some sources, prescriptions for testosterone replacement therapy (TRT) have as much as tripled in the past 10 years, but not without controversy.
Estradiol (E2) is a metabolite of testosterone and plays a role in male sexual development in early life as well as adult male sexual function in later life.17 Estradiol has also been shown to play an important role in maintaining bone density in men.18 In a 2012 case study of a 17-year-old with an aromatase deficiency, bone growth was preserved when the young man received estrogen therapy.19 Estradiol can also influence mood and cognition in men. For example, estradiol has been associated with increased serotonin binding receptor activity in men, which may improve depressive symptoms.20 In a 2014 study of 400 men on testosterone therapy, men with elevated serum estradiol and testosterone levels greater than 300 ng/dL reported better libido than those with low estradiol levels (P<0.01).21
Hormone Testing
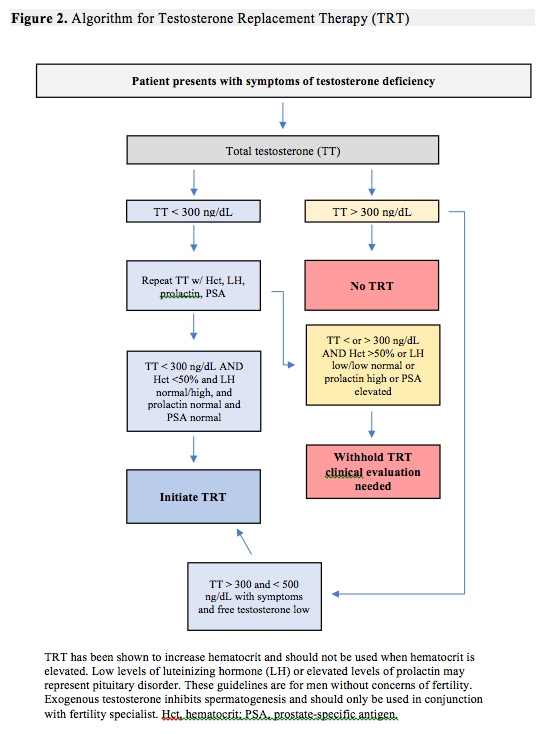
A review of androgen testing methods in men is beyond the scope of this article but, it's worth noting a few studies regarding the use of salivary vs serum levels of testosterone. Hypogonadism is defined in the 2018 Endocrine Clinical Practice Guidelines as consistently low serum testosterone levels.22 However, authors of a 2014 study of 104 men and 91 women, published in the Annuals of Biochemistry, reported that salivary testosterone was a “reliable alternative to serum testosterone in the diagnosis and management of androgen disorders and assessment of androgen status in clinical research.”23 To complicate matters, in a 2018 study of 39 women with hyperandrogenism, 88% of women with elevated serum testosterone levels showed normal salivary levels of testosterone. The authors recommend obtaining serum testosterone levels as a part of a standard endocrine workup.24 There are several laboratories that will test saliva, urine, and serum for levels of male and female hormones. This article will only review serum hormone recommendations when evaluating hypogonadism using serum levels. An algorithm for testing has been included in this article (Figure 2).
Total Testosterone
As previously mentioned, is the amount of free testosterone plus the amount bound to SHBG and albumin. The 2010 Endocrine Society Guidelines define androgen deficiency as “signs and symptoms of low testosterone (eg, decreased libido, decreased erections, decreased energy, decreased physical stamina, decreased lean muscle mass) in the setting of unequivocally low morning serum T levels of less than 300 ng/dL on 2 separate occasions.”25 If a patient has symptoms of low testosterone and the level is between 300 and 500 ng/dL, then free and weakly bound testosterone should be checked. If free testosterone is low then TRT should be considered after reviewing the benefits and risks for that individual patient.
Free Testosterone
This measures the amount of unbound testosterone. Considering that SHBG levels tend to change with obesity and age and might affect the level of free serum testosterone, it might be valuable to consider this test, especially if total testosterone is normal yet the man presents with obvious symptoms of hypogonadism.26
Weakly Bound Testosterone
This is the amount of testosterone bound by albumin. The test called “testosterone, free and weakly bound, with total testosterone” would cover all 3 of the measurements needed for most clinical practice situations. This combination is found in the major conventional laboratory companies such as LabCorp (Test #070282).
Sex Hormone–Binding Globulin (SHBG)
SHBG should be measured in older individuals because levels tend to increase with age.7 It is estimated that SHBG increases by as much as 50% by age 70. In addition, individuals with lower levels of SHGB (eg, obese persons) have been shown to have an increased risk of developing metabolic conditions such as type 2 diabetes.27 There is evidence that some metabolic conditions in obese men might be secondary to the reduction of SHBG. The degree of decline in SHBG seems to follow the degree of obesity, yet free testosterone is often normal in these men.28 If the SHBG is abnormal, a free and weakly bound serum testosterone level may help you make the decision to initiate TRT (eg, free testosterone is low).
Luteinizing Hormone (LH)
LH is made in the pituitary gland and stimulates the production of testosterone. Low levels of LH with low levels of testosterone might suggest a pituitary tumor or mass (secondary hypogonadism).
Prolactin
If a man presents with symptoms of decreased libido and infertility and has elevated levels of serum prolactin and low serum testosterone, a pituitary mass must be ruled out, especially if he also is experiencing decreased vision (caused by compression of the optic nerve).
Estradiol (E2)
Estradiol free, equilibrium dialysis, with total estradiol is the recommended testing.
Hematocrit and Hemoglobin
Testosterone has been shown to increase hemoglobin and hematocrit. Therefore these should be screened prior to initiation of TRT.29 In a review of 51 studies, the use of TRT increased hemoglobin on average by 0.80 g/dL and hematocrit by 3.18%. Injectable forms of TRT appear to cause the greatest increase compared to other forms of TRT.30
Prostate Specific Antigen (PSA)
PSA is used as a marker for prostate cancer; levels equal to or greater than 4.0 ng/mL require further investigation to rule out cancer. To date, there is no evidence showing that TRT increases the risk of prostate cancer. The use of transdermal TRT has not been shown to significantly raise PSA levels; however, small increases have been observed in men using the injectable form of TRT. In a meta-analysis of over 700 patients using TRT, there was no significant change in PSA levels in men using TRT when compared to controls (P<0.001). In the group using intramuscular TRT, there was a slight but significant increase of 0.271 ng/mL (P=0.001).31
Benefits and Risks of Testosterone Replacement Therapy
The most common documented benefits of TRT include stimulation of erythropoiesis (increased production of red blood cells), increased libido, and improved bone density, muscle mass, erections, and in some cases, mood and cognition.32,33 However, other studies and reviews are mixed regarding the benefits of TRT. In a review of 156 controlled trials between 1950 and 2016, authors concluded that TRT provided no benefit for cardiovascular risk, sexual function, mood, or cognition.34 In October of 2015, an international group of researchers gathered to draft a consensus statement on testosterone deficiency and treatment. One of the conclusions from the conference was that TRT is “effective, rational, and evidence based.”35 The following section reviews the benefits of TRT on sexual function, bone density, muscle mass, cardiovascular health, erythropoiesis, mood, and cognition.
Sexual function
The benefits of TRT for male sexual function have been mixed in the literature. In a 2016 study published in the New England Journal of Medicine involving 790 men aged 65 or older, men whose testosterone levels rose to mid-range after 1 year of TRT had moderate increase in sexual activity, increase in sexual desire, and improvement in erectile function (P<0.001).36
Bone density
Low levels of testosterone in men have been shown to be associated with osteoporosis.37 Interestingly, one published report found that older men with low bioavailable estradiol and high SHBG had an increased risk for bone fractures.38 In a study of 211 men over the age of 65 who had been diagnosed with low testosterone, TRT led to a significant increase in spine and hip bone mineral density and bone strength compared to placebo (P<0.001). The initial dose of 5 g per day of 1% AndroGel was adjusted to keep the total testosterone range within normal range. Levels of serum estradiol also increased as a result of the TRT.39
Muscle mass
Research has demonstrated that obese men have lower levels of free and total serum testosterone compared to lean men.30 In a review of literature published in 2014, the use of TRT in hypogonadal men reduced fat mass, increased lean body mass (LBM), and reduced both waist circumference and BMI.40
Erythropoiesis
Testosterone replacement therapy has been shown to increase hemoglobin and hematocrit, an effect that appears to be dose-dependent. The increase in erythropoiesis is greater in older men (ages 60-75). For example, 125 mg of weekly intramuscular injections of testosterone for 12 weeks in older men increased levels of hematocrit by 75% (P≤0.0001).32,41
Cardiovascular events
According to a 2015 position statement in Mayo Clinic Proceedings, there is no evidence that use of TRT increases cardiovascular risks.42 In 2014, the European Medicines Agency made a statement that there was no evidence that the use of testosterone as a medicine increased the risk of heart problems.43 In a review of over 1,000 men in clinical trials using TRT, increase in hematocrit was the most common side effect; no significant increase in cardiovascular events was found (CI=95%).44 In a 2014 meta-analysis reviewing over 60 clinical trials, increased risk of cardiovascular events was dependent on the method of delivery of TRT. Overall, when patients used transdermal and intramuscular injections, no increased risk of cardiovascular events was noted. However, the use of oral TRT significantly increased the risk of cardiovascular events (P<0.015).45
Mood and cognition
In an 8-month study of 106 men with diagnosed hypogonadism and symptoms consistent with low testosterone including depression and loss of concentration, no significant increase was noted in PSA. No significant changes in depression were found, but improvement in cognition was reported (P=0.94).46 In a 30-week trial of 184 men using 1,000 mg intramuscular testosterone undecanoate 3 times during the trial, there was significant improvement in depressive mood (P=0.003) and improvements in the Aging Males’ Symptoms (AMS) scale (P<0.001). The AMS is a tool used to evaluate quality of life in older men. Included in this study were men with low testosterone levels and metabolic syndrome.47 Metabolic syndrome in itself has been linked to depression in men,48 so addressing this condition with lifestyle interventions is very important.
Contraindications for testosterone replacement therapy
Testosterone replacement therapy is contraindicated for men with the following conditions: breast or prostate cancer; the presence of a nodule in the prostate; PSA level above 4.0 ng/mL (3.0 ng/mL for men with high risk of prostate cancer); desire for fertility; lower urinary tract symptoms; benign prostatic hypertrophy (BPH); severe obstructive sleep apnea; and a hematocrit level of greater than 50%.23,34
Method of Delivery
Gels and creams
Bioidentical compounded formulas usually come in 1 mg/mL to 200 mg/mL and are typically applied each morning. Some compounding pharmacies can ship to patients in various states. Many commercial formulas are available including Axiron, Androgel, Fortesta, Vogelxo, and Testim. The potential of transferring hormone to other individuals during close contact is a consideration and patients should be educated on this potential problem.
Injections
The two most common forms of injectable forms of TRT are testosterone enanthate or cypionate. Typical dosage schedules for testosterone cypionate are 100 mg weekly or 200 mg every 2 weeks. Testosterone enanthate is long-acting and administered in 200 mg/mL dose every 2 weeks. Injectable forms of testosterone will result in higher than normal levels of hormone within 24 hours of injection, which gradually return to baseline.7 Some men do not like the idea of injections, so all options should be discussed. The cost of this method is attractive to some patients.
Transdermal patch
Testosterone patches need to be applied every 24 hours and the site of application rotated. Androderm, the commercial product, comes in 2.5 mg, 5.0 mg and 7.5 mg per patch applied daily. The most common side effect is local skin irritation and is reported to occur in 28% of individuals using this form of delivery.49
Nasal testosterone
Natesto is the only available nasal testosterone and comes in pumps, with 11 mg per pump. Patients apply each dose per nostril 3 times daily. Common side effects after using Natesto for 90 days are epistaxis and nasopharyngitis.50
Cost
On average, in Arizona, a 30-day supply (30 g) of 100 mg bioidentical testosterone ranges from $40 to $90. The cost of the intramuscular forms range from $18.45 to $23.46 per month. The monthly cost of Androderm is around $195. The monthly cost of these commercial formulas can range from $268.25 to $630.78. A 1-month supply of Natesto is around $700.51
Aromatase Inhibitors
Anastrozole, a nonsteroidal aromatase inhibitor, reduces levels of circulating estrogens and is approved by the FDA for use in hormone-related breast cancer in women. The standard dose of anastrozole is 1 mg per day.52 Anastrozole has been shown to increase levels of serum testosterone while reducing circulating estradiol. In a study of 37 men over age 62, the group of 11 men using 1 mg of anastrozole twice weekly showed a significant increase in bioavailable and total testosterone, with decreased levels of estradiol (P<0.001). However, PSA levels increased significantly in this group compared to the group using 1 mg of anastrozole daily and the placebo group (P=0.031).53 An article in the New England Journal of Medicine in 2013 reported that men given an aromatase inhibitor (AI) with TRT had impaired sexual function compared to men who received TRT alone.54 In a study of 69 men over age 60 with borderline-to-low serum testosterone, 1 mg anastrozole per day for 1 year reduced bone mineral density of the spine (P=0.0014).55 To my knowledge, at the time of this article, no long-term studies on the use of anastrozole in men receiving TRT have been published, so it is important to monitor PSA, bone density, and sexual function in men who are using this medication.
Use of 5α-reductase Inhibitors
There are 3 types of 5-AR isoenzymes responsible for cell growth: type 1 (5-AR1), type 2 (5-AR2), and type 3 (5-AR3). Finasteride, a 5-AR enzyme inhibitor, inhibits 5-AR1. Finasteride was approved by the FDA in 1992 for the treatment of BPH.56 A study published in 2003 in the New England Journal of Medicine reported a 24.8% reduction in low-grade prostate cancer with the use of finasteride (P<0.001). However, the finasteride group had a 27% increased risk of developing high-grade prostate cancer compared to the placebo group (P<0.001).57 Dutasteride, a 5-AR1 and 5-AR2 inhibitor, was found to reduce the risk of low-grade prostate cancer by 23% (P<0.001) in a study involving over 6,000 men. In the dutasteride trial, the incidence of high-grade prostate cancer during the 4 years of the study did not increase significantly (P=0.15). However, 29 of the men using dutasteride were diagnosed with high-grade prostate cancer, compared to only 1 in the placebo group.58 If physicians are using finasteride and dutasteride, it is the physician’s responsibility to discuss the possible side effects of this medication, including the potential increased risk of high-grade prostate cancer.
Summary
The decision to use any type of HRT should be undertaken with open and honest dialogue with each of our patients. Potential benefits and risks must be explained and proper followup during the replacement period must be incorporated into clinical practice. Baseline laboratory markers and appropriate evaluation of medical history should be a consistent part of clinical practice. Initiating TRT when total serum testosterone is over 300 ng/dL, especially when there is an absence of hypogonadal symptoms, is not a medical practice standard. When initiating TRT for symptomatic men with total testosterone levels between 300 and 500 ng/mL, it is important to document low free testosterone at baseline. In a TRT summary published in 2013, this author recommended a “testosterone therapy test” for patients treated with TRT when their total testosterone levels are borderline low. This test evaluates the patient after 3 months of TRT. If the patient is showing improvement, continue with TRT. If the patient’s symptoms are not improving, stop TRT and look for other underlying causes of his “hypogonadal” symptoms.59 This is consistent with the naturopathic principle “identify and treat the cause.”






