Abstract
Covid-19 has become a global pandemic affecting tens of millions of people worldwide and resulting in the death of well over 1 million people as of this writing. The pathophysiology of the disease is a complex one that engenders detrimental effects on the immune system. While vaccines against the SARS-CoV-2 virus are emerging, there remains an unmet need for safe, cost-effective, and readily available treatments. In the absence of direct evidence relating to Covid-19, the goal of this article is to review the key preclinical and clinical data that support immunomodulatory properties of natural health supplements, such as North American ginseng (NAG; Panax quinquefolium), vitamin D, and zinc, as well as lifestyle factors. We highlight the molecular mechanisms by which these supplements may modulate innate and adaptive immunity. In addition, we review existing evidence from key clinical trials on how these supplements can prevent/combat upper respiratory tract infections.
Introduction
Coronaviruses, which are positive-stranded ribonucleic acid (RNA) viruses, have earned their name based on the presence of a crown of extended spike proteins on their surface.1 Seven coronaviruses that can infect humans have been identified to date. The first 2 human coronaviruses, namely HCoV-229E and HCoV-OC43, were identified in the mid-1960s as pathogenic to humans following their isolation from infected humans who were experiencing common cold symptoms.2–4 More recent outbreaks in the early 2000s allowed identification of 4 additional coronaviruses: SARS-CoV-1, HCoV-NL63, HCoV-HKU1, and MERS-CoV.1 Severe acute respiratory syndrome (SARS) coronaviruses primarily act by attacking the epithelial cells in the respiratory tract, which may cause diffuse alveolar damage.5 Patients present with flu-like symptoms including cough, fever, chills, and malaise.6 Infiltration of immune cells, including macrophages, lymphocytes, and neutrophils, has also been observed following histological examination of the lungs of SARS patients.5
On December 31, 2019, China reported to the World Health Organization (WHO) an outbreak of viral pneumonia cases linked to a seafood market in Wuhan City. Etiology of the outbreak, which subsequently expanded to become a global pandemic known as coronavirus disease 2019 (Covid-19), was identified as a new coronavirus, the SARS-CoV-2.7,8 Phylogenetic studies have shown that SARS-CoV-2 spike (S) glycoprotein sequences are highly homologous to those of bat coronaviruses, suggesting bats may be the intermediate host that facilitated human transmission.9 In addition, SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) receptor as the dominant mechanism of cell entry and the serine protease TMPRSS2 for S protein priming.10
Impact of Coronaviruses on the Innate and Adaptive Immune Response
The immune system has been found to be negatively impacted in SARS patients. Immunohistochemical analysis of spleens of SARS patients has revealed a reduction of ~50% in natural killer (NK) cells, 40% in macrophages, 80% in dendritic cells, and 91% in B cells, as well as a 70% to 80% reduction in T cell counts, compared to normal controls.11 Consistent with this data, a number of mechanisms have been reported regarding how coronaviruses can antagonize innate immune sensing, which is the first line of antiviral defense.12 SARS-CoV-1 nonstructural protein 1 (nsp1) has been shown to inhibit interferon (IFN) signaling by abrogating phosphorylation of STAT1.13 SARS-CoV-2 has been shown to act in a similar manner. SARS-CoV-2 viral proteins ORF6 and ORF8, as well as its nucleocapsid proteins, have been shown to inhibit type I interferon signaling (IFN-β), and prevent induction of IFN-stimulated genes.14 In the context of disease progression, a delayed IFN-I response has been shown to correlate with disease severity and lethal pneumonia in SARS-CoV-1 and MERS-CoV mouse models.15,16
While coronaviruses can inhibit IFN signaling as mentioned above, they can also reprogram infected cells to promote expression of proinflammatory cytokines, which appears to contribute to pathogenesis. In fact, SARS-CoV-1 can trigger activation of the NLRP3 inflammasome, leading to induction of IL-1β.17 In addition, peripheral blood mononuclear cells in Covid-19 patients were found to have enhanced expression of IL-6 and IL-18.18 This data provide insight into the mechanisms by which SARS-CoV-2 may generate the cytokine storm in Covid-19 patients.
SARS-CoV-2 infection is less frequent in children under 9 years of age with a prevalence rate ranging between 0.5% to 0.9% based on reports from Italy and China, respectively.19 The infection has also been noted to be less severe in children compared to adults. This may be due to the fact that lymphocyte counts, especially NK cells, are higher in heathy children compared to healthy adults, perhaps due to more frequent viral infections in childhood and trained immunity.19 In the context of innate antiviral immunity, NK cells are essential players where they act as first responders to restrain viral replication while the adaptive immune response builds up. Indeed, early recruitment of CD4+, NK T cells, NK cells, and macrophages was detected in the lungs of a SARS-CoV-1 mouse model following the first wave of chemokine production.20 Furthermore, in mice depleted of both CD4+ and CD8+ T cells (ie, lacking T-cell and antibody responses), the innate immune response was still able to control SARS-CoV-1 infection and replication.20 The impact of SARS-CoV-2 on innate immunity is profound. Total numbers of NK cells, CD4+ T cells, CD8+ T cells, and B cells are drastically reduced in Covid-19 patients.21–23 In addition, NK cell numbers inversely correlated with Covid-19 disease severity.21 Notably, NK cell function was also found to be exhausted in a mechanism that was attributed to increased expression of NK cell inhibitory receptor NKG2A in Covid-19 patients. This was associated with reduced expression of CD107a (a functional NK cell marker), IFN-γ, IL-2, granzyme B, and TNF-α.21 Importantly though, total numbers of CD8+ T cells and NK cells were restored in patients convalescing after therapy, together with reduced expression of NKG2A. These results suggest that SARS-CoV-2 pathogenesis involves inhibiting antiviral innate immunity.21
Collectively, these data support use of vitamin D supplementation to strengthen the immune system against foreign pathogens.
Covid-19 has also been shown to have a drastic impact on the adaptive immune response. Lymphopenia is a key feature of Covid-19 disease, affecting CD4+ (T helper cells) and CD8+ (cytotoxic cells), and is associated with severe disease.24 Counts of naïve T helper cells (CD3+CD4+CD45RA+) were found to be increased, whereas T helper cells (CD3+CD4+) and memory T helper cells (CD3+CD4+CD45RO+) were decreased in severe Covid-19 cases compared to non-severe ones.25 Consistent with this observation, total numbers of CD4+ T helper 1 (Th1) cells, as well as IFN-gamma production by these cells, were lower in Covid-19 patients with severe disease compared to patients with moderate disease.26 On the other hand, SARS-CoV-2-specific CD4+ T cells have been detected in Covid-19 patients, and these predominantly generated effector and Th1 cytokines, although Th2 and Th17 cytokines (IFN-gamma, TNF-alpha, and IL-2) were also produced.27 Furthermore, increased levels of highly proinflammatory CCR6+ Th17 cell subsets in CD4+ T cells have also been described in severe Covid-19 disease.23
It remains unclear what the underlying mechanisms of Covid-19-induced lymphopenia are. However, it’s been postulated that the SARS-CoV-2 may directly infect lymphocytes, resulting in apoptosis, or cause direct damage to lymphatic organs. Other proposed mechanisms include pro-inflammatory, cytokine-mediated lymphocyte deficiency and elevated blood lactic acid levels in Covid-19 patients, which can inhibit lymphocyte proliferation.28 SARS-CoV-2 also appears to reprogram terminally differentiated T cells and exhausted T cells in Covid-19 patients to express high levels of the inhibitory receptors PD1, TIM3, LAG3, CTLA4, NKG2A, and CD39, which may further contribute to lymphopenia.29 It is also plausible to speculate that exhaustion of NK cells may contribute to exhaustion of the T helper adaptive response because early production of IFN-gamma by NK cells promotes differentiation of naïve T cells into Th1 cells. Lack of IFN-gamma may, therefore, serve as a mechanism to suppress the T-helper pathway. Collectively, it is evident that SARS-CoV-2 exerts its pathogenicity through different weakening aspects of the immune system.
Modulation of the Immune System by Natural Health Supplements
The immunopathological features of Covid-19 patients described above highlight the degree of immune damage caused by the SARS-CoV-2 virus. Therefore, therapeutic strategies that reinforce the immune system are needed. Immunomodulatory effects of natural health supplements such as North American ginseng (NAG; Panax quinquefolium) polysaccharides, vitamin D, zinc, and antioxidants have been previously described by other research teams, and will be discussed in this section.30–33
Preclinical data of North American ginseng polysaccharides
Ginseng extracts have been traditionally used in Asia to prevent and treat various diseases. The water-soluble carbohydrate fraction of ginseng extracts contains polysaccharides and oligosaccharides that have been shown to enhance macrophage activation, increase anti-complement activity and IFN-gamma and TNF-α production, and promote phagocytosis and NK cell function.34–38
The immunomodulatory activity of CVT-E002®, an aqueous polysaccharide/oligosaccharide extract derived from NAG, was first described in 2001 by Wang et al. Wang et al showed that CVT-E002 can stimulate proliferation of B cells in mouse spleen, enhance macrophage activation, and increase expression of IL-1, IL-6, and NO, and increase IgG production in vivo and ex-vivo in mice.30 Another group corroborated these findings in murine macrophage and splenocyte cells.39 Of note, antioxidant properties of alkali-extractable polysaccharide fractions in NAG have also been documented.40
NAG root extracts have also been shown to increase expression of TNF-α in cultured human peripheral mononuclear cells and rat alveolar macrophages.41 Notably, polysaccharide-free methanol extracts containing ginsenosides could not stimulate TNF-α expression, although they have been shown to suppress lipopolysaccharide (LPS)-induced macrophage NO and TNF-α production.41,42
Induction of TNF-α mRNA expression by NAG root extracts was also described through in vitro studies by Zhou et al in human peripheral blood mononuclear cells.43 Ex vivo studies in mice have also shown that CVT-E002 significantly increased Concanavalin-A-induced IL-2 and IFN-γ production in spleen cells in a dose-dependent manner.44 Importantly, CVT-E002 has been reported to increase total NK cell counts, as well as life span of mice bearing a tumor of viral origin (erythroleukemia), and also reduce rates of moderate-to-severe acute respiratory infections in patients with chronic lymphocytic leukemia (CLL).45,46 Moreover, CVT-E002 injections in normal, infant (preweaned) mice were found to significantly increase and sustain NK and granulocyte cell counts even after the agent was withdrawn.47 Indeed, dietary supplementation with an extract of NAG in adult and juvenile mice was shown to increase NK cell numbers in the spleen and bone marrow.48,49 Furthermore, incubation of PBMCs and NK cells with various concentrations of CVT-E002 resulted in a dose-dependent activation of peripheral blood mononuclear cells (PBMCs), causing increases in IL-1α, IL-1β, IL-6, and IL-8 levels.
In the presence of CVT-E002-activated PBMCs and influenza virus, CVT-E002 activated NK cells, suggesting a mechanism dependent on monocyte activation.50 The antiviral properties of CVT-E002 have been attributed to induction of human monocyte-derived dendritic cell maturation, which results in increased memory T cell activation and Th1-type cytokine response.51 The pro-immune properties of aqueous NAG extracts have also been documented in a rat model of Pseudomonas aeruginosa where coadministration of the extracts along with tobramycin stimulated the pro-inflammatory response and reduced lung infection.52
From a mechanistic standpoint, CVT-E002 stimulated IL-8 production from cells expressing human toll-like receptor 4 (hTLR 4) and MD2-CD14 in a dose- and time-dependent manner. CVT-E002 also stimulated cells expressing hTLR 2 in a dose-dependent manner, which suggests that CVT-E002’s actions on the innate immune response are likely mediated via TLR 2.53 Moreover, transcriptome analysis of human PBMCs treated with CVT-E002 revealed activation of the MAPK (ERK-1/2), PI3K, p38, and NF-κB pathways, suggesting their potential implication in the immunostimulatory response.54
Clinical efficacy of North American ginseng polysaccharides
The clinical efficacy and safety of NAG extracts, including CVT-E002, have been assessed in preventing upper respiratory tract infections in several clinical trials that will be discussed in this section.
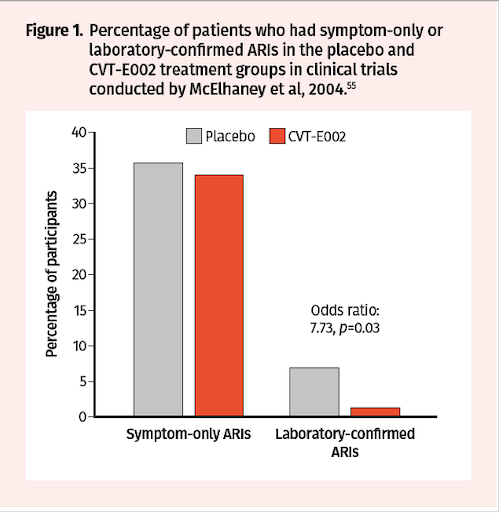
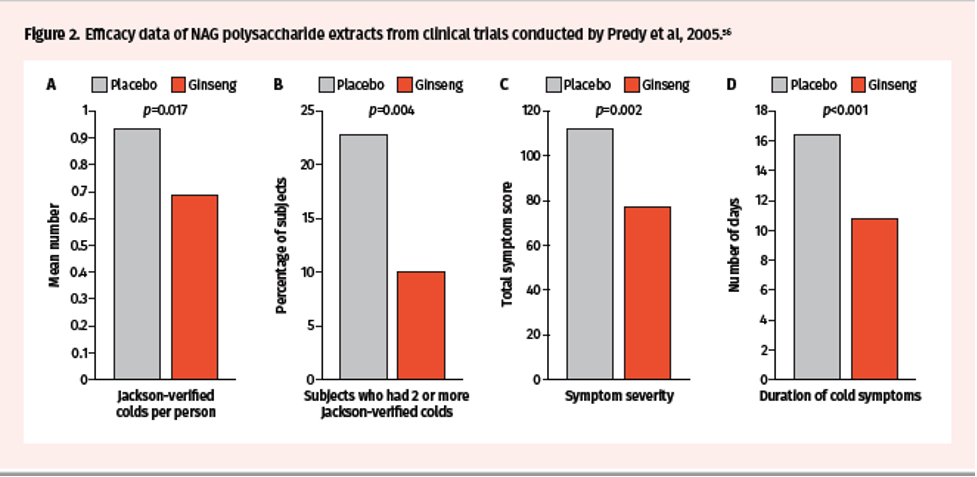
Two randomized, double-blind, placebo-controlled trials were conducted in the early 2000s by McElhaney et al over 2 periods—8 weeks and 12 weeks—to assess impact on incidence of acute respiratory illness (ARI) during the influenza season.55 Trial participants received CVT-E002 200 mg orally twice daily or placebo and were monitored for evidence of new respiratory symptoms twice weekly. While there was no statistical difference in the incidence of ARI (defined by symptoms alone) between treatment groups, the incidence of laboratory-confirmed influenza was higher in the placebo group (7/101) compared to the CVT-E002 group (1/97; odds ratio: 7.73, P=0.03) (Figure 1). The efficacy of NAG extracts containing poly-furanosyl-pyranosyl-saccharides was also investigated in randomized, double-blind, and placebo-controlled clinical trials conducted by Predy et al in 2005.56 Over a period of 4 months, a total of 323 subjects received ginseng extracts 200 mg orally twice daily or placebo and were monitored for Jackson-verified colds, symptom severity, and total duration of symptoms and colds. The mean number of Jackson-verified colds per person was lower in the ginseng vs placebo groups (0.68 vs 0.93; 27% reduction, 95% CI 0.04–0.45, P=0.017) (Figure 2A). In addition, the proportion of subjects who had 2 or more Jackson-verified colds was significantly lower in the ginseng vs placebo groups (10% vs 22.8%; difference 12.8%, 95% CI 4.3–21.3, P= 0.004) (Figure 2B). Importantly, there was a reduction in total symptom scores in ginseng vs placebo (77.5 vs 112.3; 31% reduction, 95% CI 1.2–2.0, P= 0.002) and total duration of symptoms (10.8 vs 16.5 days; 35% reduction, 95% CI 1.3–2.0, P<0.001) (Figure 2C-D).
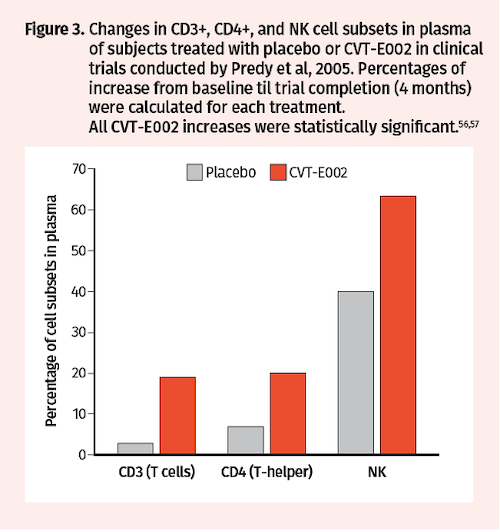
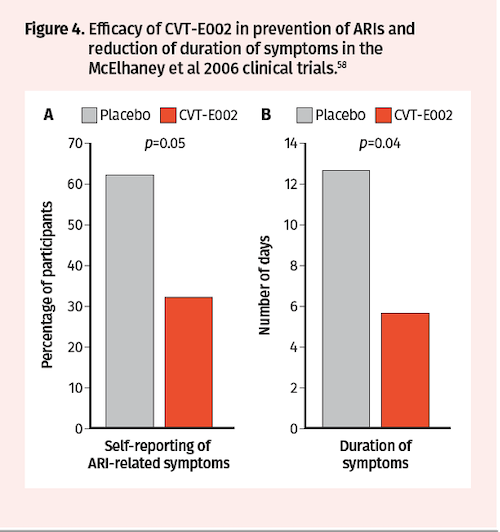
Subsequent analysis of blood samples drawn from a subset of Predy et al trial participants at baseline and after treatment revealed that CVT-E002 vs placebo increased the proportion of CD3+ T lymphocytes (19% vs 3%), CD4+ T helper cells (20% vs 7%), and NK cells (63% vs 40%) (Figure 3).57 Additional randomized, double-blind, placebo-controlled clinical trials were conducted by McElhaney in 2006 to assess CVT-E002 efficacy in prevention of ARIs in 43 community-dwelling adults over a period of 4 months. Consistent with the Predy et al 2005 trial results, McElhaney et al found significantly fewer subjects with acute respiratory illnesses in the CVT-E002 vs placebo groups (32% vs 62%; P=0.05) (Figure 4A). Moreover, duration of symptoms was also significantly reduced with CVT-E002 treatment (5.6 vs 12.6 days; P=0.05) (Figure 4B).58
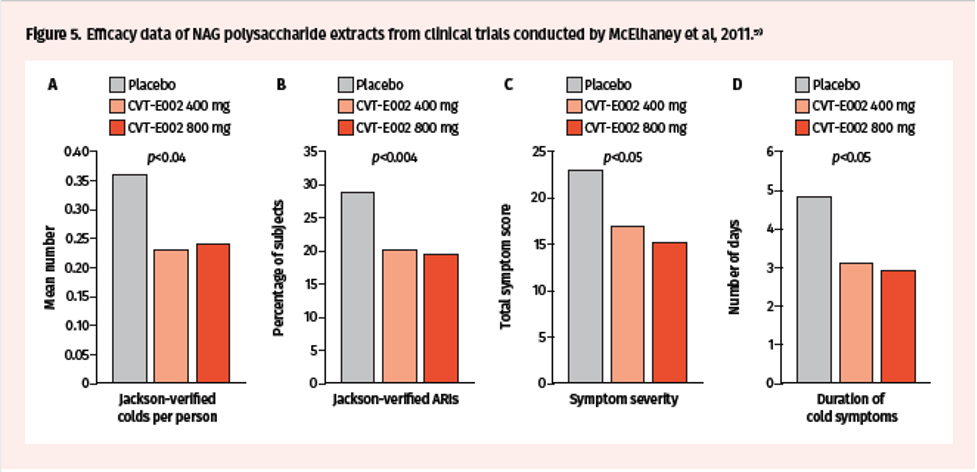
Lastly, a multicenter, randomized, double-blind, placebo-controlled trial conducted by McElhaney et al in 2011, in 783 influenza-vaccinated community-dwelling adults, assessed efficacy of 400 mg and 800 mg CVT-E002 in preventing ARIs.59 This trial showed a reduction in the number of ARIs per subject with CVT-E002 treatment (placebo vs 400 mg vs 800 mg: 0.36 vs 0.23 vs 0.24) (Figure 5A). Moreover, the incidence of Jackson-confirmed ARIs was 28.9%, 20%, and 19.4% in the placebo, 400 mg, and 800 mg groups, respectively (Figure 5B). There was also a reduction in the total symptom score (49.6 vs 43.3 vs 38) and total ARI duration (4.87 vs 3.13 vs 2.93 days) (Figure 5C-D). Importantly, CVT-E002 appeared to provide further reduction of cold and flu symptoms to that provided by the influenza vaccine in the trial participants.
Regarding safety, it is noteworthy that in all the aforementioned clinical trials, NAG extracts were well tolerated, and there was no statistically significant difference in the adverse event profile between placebo and treatment groups.55,56,58,59
Based on the aforementioned preclinical and clinical data of NAG extracts, a tentative mechanism of action for immunomodulatory properties is one that entails activation of toll-like receptors on macrophages and dendritic cells, which subsequently stimulates both innate and adaptive immunity to viral infections. Activation of antiviral cytokines further activates dendritic cells, macrophages, NK cells, killer T cells, and helper T cells. Viral particles are ultimately removed by antibodies or macrophages, and infected cells are destroyed by killer T cells or NK cells.
Vitamin D
Vitamin D is an immunomodulatory hormone that regulates calcium and phosphate metabolism.31 Historically, vitamin D3-rich cod liver oil and sunlight exposure were used as treatments for tuberculosis. Vitamin D exerts its physiologic functions by binding the vitamin D receptor (VDR), and several studies have shown its immunostimulatory properties. Indeed, vitamin D can enhance innate immunity by enhancing expression of antimicrobial peptides such as cathelicidin and defensins; can reduce production of pro-inflammatory cytokines such as TNF-α and IFN-γ, which may in turn lessen the intensity of the cytokine storm in Covid-19 patients; inhibits influenza and rotavirus replication; enhances expression of antioxidation genes; and promotes induction of Treg cells, which can additionally help inhibit inflammation.60
Results from a prospective cohort study in the UK showed that higher 25-hydroxyvitamin D levels were associated with lower risk of all-cause, cardiovascular disease, and cancer mortality.61,62 In addition, there was a negative correlation between vitamin D levels and prevalence and mortality rates of Covid-19.61 In Spain, Italy, and Switzerland, vitamin D levels were found to be severely low in the aging population, which is the most vulnerable group to bad outcomes of Covid-19.62 Data from The Irish Longitudinal Study on Ageing (TILDA) similarly showed that the highest rates of vitamin D deficiency are observed in elderly people in Ireland (30% of those aged >70 years and 47% of those aged >85 years).63 Vitamin D levels were also found to be severely low in participants who were obese or had preexisting lung conditions, both of which are groups at high risk of Covid-19.63
Importantly, results from a systemic review and meta-analysis of randomized clinical trials of vitamin D2 or D3 supplementation showed that it is associated with reduced risk of acute respiratory tract infections (odds ratio 0.88; 95% CI 0.81–0.96; P<0.001).64 Furthermore, subgroup analysis showed that protective effects of vitamin D against infections are observed in participants who received vitamin D daily or weekly without further bolus dose supplementation.64 Meta-analysis of 8 observational studies also revealed an increased risk of developing pneumonia in subjects who had 25-hydroxyvitamin D deficiency (<20 ng/mL; odds ratio 1.64; 95% CI 1.00–2.67).65 Consistent with this data, secondary analysis of the Third National Health and Nutrition Examination Survey, a probability survey conducted in the US in 18,883 participants, revealed that serum 25-hydroxyvitamin D levels were inversely correlated with self-reported recent upper respiratory tract infections (URTIs).66 Infectious symptom and antibiotic use were also reduced with daily vitamin D supplementation in randomized clinical trials of participants who have antibody deficiency or increased susceptibility to contracting respiratory infections.67
Collectively, these data support use of vitamin D supplementation to strengthen the immune system against foreign pathogens. Efforts are currently underway to assess the impact of lifestyle and diet, including vitamin D supplementation, on Covid-19 severity and SARS-CoV-2 transmission.68,69 Given that SARS-CoV-1 has been shown to reduce expression of ACE2 receptors, it has been postulated that SARS-CoV-2 may act in a similar fashion.10,70 This in turn precludes degradation of angiotensin II, and results in increased vasoconstriction, inflammation, and thrombosis in Covid-19 patients.71 Importantly, 1,25-dihydroxyvitamin D3 (calcitriol), the active form of vitamin D, has been shown to increase ACE2 expression in microglial cells.72 It remains to be determined whether this phenomenon also occurs in Covid-19 patients as a mechanism by which vitamin D may counteract SARS-CoV-2 infection. It’s also important to note that supplementation of vitamin D together with other agents to potentiate its effects may prove beneficial.
Given that vitamin D is an antioxidant and oxidative stress is a process tightly linked to inflammation, could coadministration of antioxidants such as N-acetylcysteine be used as a preventive measure for infections? Evidence suggests that antioxidants such as N-acetylcysteine can reduce oxidative stress and inflammatory lung damage, frequency of influenza-like episodes, severity, and length of time confined to bed.33,73 Additional research is needed to determine if combining vitamin D together with other antioxidants could abrogate potential oxidative stress in Covid-19 patients, or limit the damage caused by the cytokine and/or bradykinin storm.
Zinc and zinc ionophores
Zinc is an essential mineral element for many cellular processes such as gene expression, DNA replication, and cell growth and proliferation. Its immunomodulatory properties have also been documented. In the early 1980s, it was shown that quercetin, a zinc ionophore, has antiviral properties against poliovirus.74 Subsequent studies confirmed that quercetin exhibits both antiviral and antioxidant activity; it can inhibit histamine release and decrease pro-inflammatory cytokines.75 Consistent with this, data from a randomized community clinical trial have shown that quercetin supplementation is associated with lower URTI severity (36% reduction; P=0.020) and URTI total sick days (31% reduction; P=0.048) compared to placebo.76
The antiviral properties of zinc have been attributed to its ability to directly inhibit isolated and purified/recombinant RNA-dependent RNA polymerase complexes. Importantly, combining zinc with pyrithione, a zinc ionophore, could inhibit SARS-CoV-1 and equine arteritis virus replication in cell culture, as well as replication of other positive-sense RNA viruses.32 Furthermore, zinc supplementation has been shown to enhance cytokine expression in human leukocyte subsets; it resulted in increased TNF-α and IL-1β expression in LPS-activated monocytes and granulocytes, and IFN-γ mRNA levels were higher in activated T lymphocytes.77
Other reports have also shown that zinc supplementation can protect the lung epithelium during inflammatory stress, whereas depletion of intracellular zinc using chelating agents enhances extrinsic apoptosis of lung epithelium.78 Zinc also protects against mechanical barrier dysfunction of the lung epithelium and degradation of cell junction proteins induced by inflammatory cytokines.79 Notably, zinc deficiency blocks antibody production, reduces NK cell activity, and decreases cytokine production by monocytes. It can also result in thymic atrophy, lymphopenia, and increased duration of infection. Conversely, zinc supplementation sustains B-cell receptor signaling, B-cell maturation, and antibody production. It also increases NK cell cytotoxic functions and wound healing.80–82
While data remains very limited as to how zinc supplementation may impact Covid-19 disease progression, a small sample study (4 patients) has shown that high-dose oral zinc salt lozenges can improve objective and symptomatic Covid-19 disease measures within 24 hours of initiation.83 It is important to note that large, randomized clinical trials remain necessary to address efficacy and whether zinc could be used in a prophylactic setting or in treatment of Covid-19.84
Impact of Lifestyle on the Immune System
In addition to diet, exercise, sleep, and stress can have a profound impact on the immune system. A meta-analysis of 30 Covid-19 studies encompassing a total of 6,452 patients revealed that diabetes mellitus was associated with mortality and severe Covid-19 disease and disease progression.85 Obesity has also been reported to be an important risk factor for intensive care unit admission, invasive mechanical ventilation, and mortality in Covid-19 patients.86,87
Regular exercise is an important factor in maintaining a healthy lifestyle. Not only does it help improve circulation, but it also depletes toxins from the lungs and the body and helps maintain a healthy weight. There is a growing body of evidence that exercise can have a positive impact on the immune system. For instance, NK cell cytotoxic activity has been shown to be 57% higher in marathon runners compared to sedentary controls.88 Physical inactivity has also been associated with lower NK cell activity.89 In addition, healthy adults who engage in moderate and regular exercise have a reduced risk of URTIs by 20%.90 Adequate sleep can also enhance the adaptive immune response by increasing antigen-specific Th cell numbers, resulting in immunologic memory.91
Conclusions
Currently, efforts are underway to uncover novel therapeutic strategies to prevent or treat Covid-19 and other potential infections. Vaccines are being fast-tracked for worldwide distribution. Natural health supplements represent an important category of compounds that have immunomodulatory potential. As described in this review article, various preclinical and clinical studies support the efficacy of these compounds in reducing the rate of infections and boosting the immune system. These compounds are easily accessible and cost-effective and generally safe to use without any serious adverse effects. Although the preceding preclinical and clinical trials have shown promising results with regards to immunomodulatory effects, the use of natural health supplements as approved treatments in a prophylactic setting or in specific treatment of Covid-19 patients still requires further clinical investigation.
Abbreviations
ACE2: angiotensin-converting enzyme 2
ARI: acute respiratory illness
CLL: chronic lymphocytic leukemia
Covid-19: coronavirus disease 2019
hTLR: human toll-like receptor
IFN: interferon
IL: interleukin
IgG: immunoglobulin G
LPS: lipopolysaccharide
NAG: North American ginseng
NK: natural killer
NO: nitric oxide
nsp1: nonstructural protein 1
PBMC: peripheral blood mononuclear cell
RNA: ribonucleic acid
S: spike
SARS: severe acute respiratory syndrome
Th: T helper
TILDA: The Irish Longitudinal Study on Ageing
TNF: tumor necrosis factor
URTI: upper respiratory tract infections
VDR: vitamin D receptor
WHO: World Health Organization
Conflict of Interest Disclosures
Will Cheng, MD, has no conflicts of interest.
Jean-Yves Dionne, BPharm, has provided consultation services for Valeant (2018), Orimed Pharma (2019-2020), Nadurel Pharma (2019), and Union Nature (2020) as scientific documentation, formulation, and teaching. Dionne has no association with and is not on the board of any pharmaceutical or natural product company.
Maxime Barakat, MD, PhD, MBA, is an employee and shareholder of Bausch Health Companies (BHC).
Acknowledgments
Assistance with writing this manuscript was provided by STA HealthCare Communications, funded by Bausch Health Canada Inc.








