Editor's note: This is Part 2 of a 2-part series on Osteoporosis. Part 1 is Making Sense of Osteoporosis Testing: What clinicians need to know to sort through screening and diagnostic technologies.
Abstract
Clinicians generally understand there is an increased risk of osteoporosis with age, menopausal status, lifestyle issues, nutrient depletions, and several comorbidities. However, there seems to be limited understanding about the growing list of medications that impact bone metabolism, decrease bone mineral density (BMD), and increase fractures. According to the Centers for Disease Control and Prevention (CDC), 73.9% of physician office visits involve drug therapy. In the United States, approximately 44% of men and 57% of women aged 65 years or more take at least 5 medications, and 12% of people in this age group take 10 or more. Given the high number of patients taking medication, clinicians will undoubtedly work with patients who are filling prescriptions that increase the risk of osteoporosis and fragility fractures. Understanding which medications increase osteoporosis and fracture risk, and their mechanisms of action, can assist healthcare providers in knowing when they need to take steps to mitigate this adverse effect or change the medication altogether. This review evaluates the damaging skeletal effects of some of the most commonly prescribed medications. Evidence-based recommendations for reversing bone loss and preventing fractures in these patients are also covered, with highlights on vitamins K and D and calcium.
Introduction
Osteoporosis is a disease characterized by decreased bone quantity and quality with a concomitant increase in fracture risk. It is the most common metabolic bone disease, with an estimated 200 million people worldwide, including 53 million Americans, either already having osteoporosis or being at high risk.2,3 In women an accelerated rate of bone loss occurs during menopause and for about 10 years after, and postmenopausal women are at the highest risk for primary osteoporosis.4,5
In a 2016 retrospective cohort study published in the journal JAMA Internal Medicine, researchers evaluated prescription drug–use patterns before and after fragility fractures in 168,133 Medicare beneficiaries who survived a fracture of the hip, shoulder, or wrist.6 They determined that 42.2% of patients who sustained a fragility fracture were taking 1 or more drugs that decrease bone density, 76% took 1 or more nonopiate medications associated with increased fracture risk, and 55.7% took 1 or more prescriptions that increase fall risk.
Yet overall there was no subsequent reduction in medications that cause osteoporosis and increase fracture risk. According to the authors, “When we further examined pre-fracture and post-fracture drug use, we observed a group of patients who stopped taking fracture-promoting drugs after fracture; however, this group was offset by other patients who initiated therapy with a high-risk drug after fracture.”
According to the CDC, 73.9% of physician office visits involve drug therapy.7 In the United States, approximately 44% of men and 57% of women aged 65 years or more take at least 5 medications, and 12% of persons in this age group take 10 or more.8 Additionally, up to 91% of patients in long-term care take 5 or more medications.9 In the United States and Canada, overall prescription use is similar, with nearly 70% of adults aged 40 to 79 years taking at least 1 prescription medication, a percentage that increases to about 83% for those aged 60 years or more.10
Given the high number of patients taking medication, clinicians will undoubtedly work with patients who are filling prescriptions that increase the risk of osteoporosis and fragility fractures. Understanding which medications increase osteoporosis and fracture risk (Table 1) can help healthcare providers know when they need to take steps to mitigate this side effect or get the patient off the medication altogether. Such medications need to be reassessed due to a changing risk/benefit over time. The mortality rate for the first year following a hip fracture in the elderly (those aged 65 years or more) is estimated to be up to 36% higher compared to age-matched controls.11 And among those who survive the first year after a hip fracture, 20% require nursing home care and 50% never return to full function.12
Table 1. Medications causing osteoporosis and fractures
| Medication Category | Examples |
| Glucocorticoids | beclomethasone, budesonide, cortisone, deflazacort (Emflaza), dexamethasone (Decadron, Dexamethasone Intensol, DexPak), fludrocortisone (Florinef), hydrocortisone (Cortef, Solu-Cortef), methylprednisolone (Medrol, Depo-Medrol, Solu-Medrol), prednisolone (Millipred, Orapred, Prelone), and prednisone (Prednisone Intensol)
|
| Acid Suppression Therapy | esomeprazole (Nexium), omeprazole (Prilosec), pantoprazole (Protonix), lansoprazole (Prevacid), and rabeprazole (Aciphex) |
| Antidepressants | SSRIs (selective serotonin reuptake inhibitors): citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac), paroxetine (Paxil), and sertraline (Zoloft) SNRIs (serotonin-norepinephrine reuptake inhibitors): atomoxetine (Strattera), desvenlafaxine (Pristiq), duloxetine (Cymbalta), andvenlafaxine (Effexor XR) |
| Aromatase Inhibitors | exemestane (Aromasin), letrozole (Femara), and anastrozole (Arimidex) |
| Androgen-Deprivation Therapy | leuprolide (Lupron, Eligard), goserelin (Zoladex), triptorelin (Trelstar), and histrelin (Vantas, Supprelin LA) |
Unfortunately, guidelines for mitigating medication-induced osteoporosis (MIO) and fracture risk do not exist for most drugs; however, general best practices still apply. The best case is to discontinue the medication or switch to 1 that does not damage bone; however, that is not always possible. The overall natural approach includes following a whole-foods, Mediterranean dietary pattern, which is associated with a 21% decrease in hip fractures (RR: 0.79; 95% CI: 0.72–0.87);13 exercising to improve strength and balance; optimizing sleep; and supplementing with nutrients shown to stop and reverse medication-induced bone loss. Additionally, individual recommendations can address the medications’ underlying mechanisms of actions that damage bone. Specific clinical recommendations are given at the end of each medication section.
Glucocorticoids
These medications include prednisone, dexamethasone, and prednisolone. Short-course and long-term prescriptions of these medications are commonly prescribed for autoimmune and inflammatory diseases such as rheumatoid arthritis (RA), polymyalgia rheumatica, asthma, inflammatory bowel disease (IBD), rashes, and chronic obstructive pulmonary disease (COPD).
Historically it has been well-accepted that long-term oral glucocorticoid (GC) use increases osteoporosis and fracture risk. Fractures may occur in 30% to 50% of patients on chronic GC therapy, most frequently in the vertebrae and femoral neck.14 An estimated 1% to 2% of the general population receives long-term GC therapy.15 In women with postmenopausal osteoporosis, the prevalence of GC use is even higher. In the Global Longitudinal Study of Osteoporosis in Women (GLOW), researchers evaluated medical records from 60,393 postmenopausal women in 10 countries and determined that 4.6% were receiving oral GCs at their baseline visit.16,17
Even small amounts of oral GCs (>2.5 mg/day over approximately 6 months) are associated with a 20% to 200% increase in risk of vertebral fractures compared to patients not taking GCs.18 One study found that for each 10-mg increase in dosage between patients, there was a 62% increase in fracture risk.19 Long-term GC therapy is common in patients with severe asthma. Compared with the nonasthma control group, when patients with severe asthma were exposed to 5 mg per day of prednisolone, they were significantly more likely to be diagnosed with osteoporosis (OR: 6.53; 95% CI: 4.63–9.21; P<0.001) or osteopenia (OR: 6.68; 95% CI: 4.28–10.43; P<0.001) and 1.65 times more likely to experience a fracture (95% CI: 1.14–2.39; P<0.001).20
In contrast to long-term use, until recently the negative impact of short-term use on bone health had been less clear. In 2017, a retrospective cohort study published in the British Medical Journal evaluated a nationwide data set of private insurance claims for more than 1.5 million US adults aged 18 to 64 years.21 The study found that over a 3-year period, approximately 20% of American adults in a commercially insured plan took oral GCs for fewer than 30 days.
The most common prescription was a 6-day methylprednisolone “dosepak,” which accounted for 46.9% of prescriptions. Among GC users, 70.5% received 1 course of treatment, 20.7% received 2 courses, and 8.8% received 3 or more courses. About half of all prescriptions were for just 5 conditions: upper respiratory tract infections (URTI), spinal conditions, intervertebral disc disorders, allergies, and nonbronchitic lower respiratory tract disorders.
Fractures were the most common complication in GC users (21 of every 1,000 users annually), and the incidence of fracture was significantly more common in GC users than nonusers. In the 5 to 30 days after GC initiation, with a median dose of 19 mg/day and a median of 6 days using steroids, the incidence rate ratio for fracture was 1.87 (95% CI: 1.69–2.07; P<0.001) for patients taking a GC compared to those not taking a GC. For the period 31 to 90 days after initiation, the incidence rate ratio declined but was still significant at 1.40 (95% CI: 1.29–1.53; P<0.001). The absolute risk of a fracture 90 days after a clinical visit was 0.51% for GC users compared to 0.39% for nonusers. White patients had a higher short-term risk of fractures than nonwhite patients (incidence rate ratio: 2.02; 95% CI: 1.81–2.26 for white patients; incidence ratio: 1.42; 95% CI: 1.14–1.77 for nonwhite patients; P=0.006).
Mechanisms of Action
While the mechanisms of action by which GCs damage bone are well-established, it’s important to understand that since fracture risk increases before changes in BMD are detected, bone density tests have limited predictive value in GC-induced osteoporosis.19 An excellent review published in 2015 in the journal Rheumatic & Musculoskeletal Diseases describes the direct and indirect effects GCs, their duration, and dose have on bone quality, quantity, and fall and fracture risk (Figure 1).22 GCs decrease osteocyte and osteoblast function while increasing osteocyte and osteoblast apoptosis. At the same time, GCs increase osteoclast proliferation while decreasing osteoclast apoptosis. These medications also affect the neuroendocrine system and calcium metabolism and damage muscle. The result of these effects is to decrease bone formation; increase bone resorption; decrease bone quality and mass; and damage muscle myofibrils to create muscle weakness, increasing fall risk. Fortunately, after discontinuing the GCs, fracture risk declines; however, whether it returns to baseline values is unclear.18,23-25
Clinical Recommendations
The 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis stratifies treatment recommendations based on whether patients are at low, moderate, or high risk for fractures.26 For every risk group, the recommendation includes supplementation with calcium and vitamin D.
For moderate- and high-risk patients, the panel recommends adding an osteoporosis medication, with an oral bisphosphonate being its preferred first-line treatment option. When a bisphosphonate is not appropriate, it recommends other medications such as teriparatide or denosumab. However, there is an important caveat for all of the recommendations. The guidelines state, “Because of limited evidence regarding the benefits and harms of interventions in GC users, most recommendations in this guideline are conditional (uncertain balance between benefits and harms).”
When deciding how to protect patients’ bones when taking glucocorticoids, clinicians should consider adding MK4 (45 mg/day) to their treatment plan. MK4 is a naturally occurring form of vitamin K2 and the only form shown to preserve bone mass in patients taking glucocorticoids27-29 and reduce fractures in postmenopausal osteoporosis.30
A 2005 study published in the Journal of Bone and Mineral Metabolism evaluated the effect of MK4 on bone mineral density in patients with glomerulonephritis taking prednisolone.27 This prospective, 12-month study randomized 12 men and 8 women to receive MK4 (45 mg/day) or no MK4 plus prednisolone. None of the patients had previously taken GCs prior to enrolling in the study. The renal diagnoses were minimal change nephrotic syndrome (n=10), membranous nephropathy (n=7), immunoglobulin A (IgA) nephropathy (n=2), and lupus nephritis (n=1).
Prednisolone treatment was initiated at 30 to 50 mg/day and then gradually reduced, with the final treatment dose being 8.9 to 11.3 mg. The cumulative dose of GC over the 12-month study was 6,576.0 to 7,625.3 mg. Baseline renal function was normal (serum creatinine <1.2 mg/dL) in all patients. Lumbar bone density was measured by dual-energy X-ray absorptiometry (DEXA) at baseline and at 6 and 12 months after starting GC therapy.
Ten patients (6 men, 2 premenopausal women, and 2 postmenopausal women, mean age 41.6+7.2 years) received 45 mg/day MK4 plus GC. Another 10 patients (6 men, 2 premenopausal women, and 2 postmenopausal women, mean age 38.5+6.4 years) received GC alone.
In this study, the addition of 45 mg/day of MK4 prevented loss of bone mass. Lumbar BMD in patients taking MK4+GC was not significantly changed at any time period, while lumbar BMD significantly decreased at 6 and 12 months in patients taking GC alone (P<0.001 for both time periods). In addition, 1 patient taking GC alone experienced a lumbar fracture, while no patients taking MK4+GC experienced a fracture.
An earlier, 2004 study showed similar results. Patients aged 15 to 57 years with glomerulonephritis treated for 2 years with prednisolone alone had a significant decrease in lumbar BMD (P=0.020), while those volunteers treated over the same time period with prednisolone plus MK4 (45 mg/day) did not have a significant change in BMD (P>0.999).28
And a third study in pediatric patients aged 4 to 14 years further supports the use of MK4 for prevention of GC-induced osteoporosis.29 In this study children who had been treated with prednisolone for more than a year had either alfacalcidol (0.03 mcg/kg/day) or alfacalcidol (0.03 mcg/kg/day) plus MK4 (approximately 2 mg/kg/day). Investigators tested lumbar BMD at baseline and after the 12-week treatment. After 12 weeks, the group receiving alfacalcidol plus MK4 showed significantly increased lumbar BMD compared to the group receiving only alfacalcidol (P=0.0029).
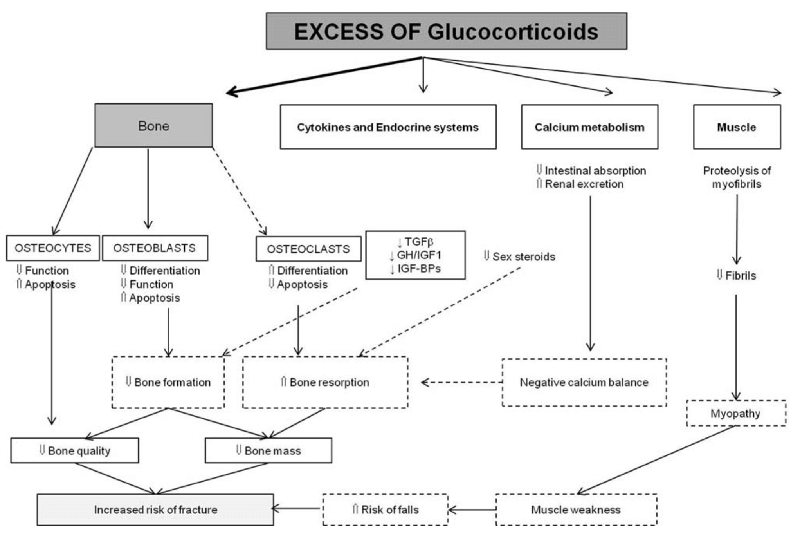
Figure 1. Glucocorticoid effects on bone.22 Abbreviations: TGFβ, transforming growth factor beta; GH, growth hormone; IGF1, insulin-like growth factor 1; IGF-BPs, insulin-like growth factor bind proteins; ↑ increase; ↓ decrease. Open Access: This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work noncommercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: http:// creativecommons.org/licenses/by-nc/4.0/
Acid Suppression Therapy
Acid suppression therapy is using proton pump inhibitors (PPI) and histamine-2 receptor antagonist (H2RA) medications that have been approved by the FDA for symptoms of gastroesophageal reflux disease (GERD), healing and maintenance of erosive esophagitis, eradication of Helicobacter pylori infections, prevention and treatment of gastric ulcers induced by nonsteroidal anti-inflammatory drugs (NSAIDs), healing of gastric and duodenal ulcers, and treatment of pathological hypersecretory conditions such as Zollinger-Ellison syndrome. PPIs are the most commonly used acid-suppression medications, and their use has been associated with increased fall and fracture risks.31
PPI drugs include esomeprazole (Nexium), omeprazole (Prilosec), pantoprazole (Protonix), lansoprazole (Prevacid), and rabeprazole (Aciphex). Fifty percent of all PPI prescriptions are written for just 1 condition: GERD.32 Acid-suppression medications are some of the most widely prescribed drugs in the world, accounting for global sales of over $11 billion.33 In the United States in 2014, pharmacies dispensed 4.325 billion prescriptions for all medications, and among those, omeprazole was the sixth most commonly prescribed drug.34 In 2012, 14.9 million patients received 157 million PPI prescriptions.35
The FDA has been aware of the increased risk of fractures from PPIs since at least 2010. In that year, the FDA warned of potential PPI-induced risk of hip, spinal, or radial fractures.36 One of the earliest studies to find a significant association between use of acid-blocking medications and fracture risk was a 2006 nested, case-control study published in JAMA.37 Researchers evaluated medical records from 192,028 patients who were taking PPIs. Patients included in the study were approximately 80% female and 20% male with a mean age of 77+9.3 years.
The crude OR for hip fracture associated with more than 1 year of PPI use was 1.82 (95% CI: 1.67–2.00; P<0.001). Since many patients are taking more than 1 medication and may have additional risk factors for falls and fractures, the crude OR may be a more accurate estimate for a patient’s risk in real-world settings. However, even when removing all potential confounders, the increased risk for hip fractures was still significant. The multivariable adjusted odds ratio (AOR) was 1.44 (95% CI: 1.30–1.59; P<0.001). For patients taking H2RA medications for more than 1 year, the AOR for hip fracture was 1.23 (95% CI: 1.14–1.39; P<0.001).
Hip fracture risk significantly increased with therapy duration and dose. After 4 years of cumulative PPI use, the OR for hip fracture was 2.17 (95% CI: 1.93–2.45; P<0.001) and the AOR was 1.59 (95% CI: 1.39–1.80; P<0.001). High-dose PPI use, defined as >1.75 times the average daily dose, was associated with greater hip fracture risk compared to non-PPI users (AOR: 2.65; 95% CI: 1.80–3.90; P<0.001). Interestingly, hip fracture risk in PPI users was stronger in men (OR: 1.78; 95% CI: 1.42–2.22) than women (OR: 1.36; 95% CI: 1.22–1.53; P=0.04 for difference between sexes).
When their analysis was restricted to patients with GERD, the AOR for hip fracture with long-term PPI therapy was 3.49 (95% CI: 1.24–9.84; P=0.02). However, in patients with GERD, H2RA use was not significantly associated with increased fracture risk (AOR: 1.21; 95% CI: 0.67–2.22; P=0.46).
A 2019 meta-analysis of prospective, randomized, case-controlled studies evaluated data from 8 studies totaling 367,068 volunteers.31 PPI use was significantly associated with an increased risk for falls compared to volunteers not taking PPIs, with an OR of 1.27 (95% CI: 1.07–1.50; I2=82%). In patients using PPIs, the risk of sustaining a fall that required hospitalization was significantly greater than for people not taking PPIs, with an OR of 1.61 (95% CI: 1.18–2.20; I2=67%). In contrast to PPIs, there was no significant association of increased fall risk in people taking H2RA medications such as cimetidine (Tagamet) and famotidine (Pepcid AC).
The use of bisphosphonates does not appear to attenuate the increased fracture risk from PPIs. A 2015 meta-analysis published in the International Journal of Clinical and Experimental Medicine evaluated data from 4 studies with a total of 57,259 patients.38 Pooled analysis demonstrated an increase in fracture risk in patients taking PPIs with bisphosphonates versus bisphosphonate-only patients (OR: 1.52; 95% CI: 1.05–2.19; P=0.025). There was no statistically significant difference in fracture risk between patients taking different types of bisphosphonates (eg, risedronate vs alendronate). To date, there are no published clinical studies evaluating the concomitant use of PPIs with other categories of osteoporosis medications.
Mechanisms of Action
As shown in Figure 2, multiple mechanisms of action are proposed to explain PPIs' effects on bone and fracture risk.39 PPIs increase the risk for multiple nutritional deficiencies, including magnesium, calcium, and vitamin B12. Decreased serum magnesium reduces osteoblast activity while increasing osteoclast activity. Decreased serum calcium can induce bone resorption by creating hyperparathyroidism. One study found that both vitamin B12 and serum ferritin significantly decreased after 1 year of taking a PPI (P<0.001 for both),40 while another found that after 3 years of taking the medication, 22.2% were deficient in vitamin B12.41 Low vitamin B12 can create hyperhomocysteinemia, which increases pre-osteoblast cell apoptosis and osteoclast activity. Reductions in vitamin B12 can lead to peripheral neuropathy, reduced muscle strength, and increased risk for falling.
Clinical Recommendations
Currently there are no published guidelines for the prevention or treatment of PPI-induced osteoporosis. The general recommendation for calcium and vitamin D is important, especially since PPIs reduce calcium absorption. Clinicians should also consider supplementing with additional nutrients that PPIs deplete. These include magnesium, calcium, iron, vitamin B12, and other B vitamins.
Monitoring serum ferritin can provide a good clinical indicator of whether more iron is necessary. Serum ferritin is the most sensitive and reliable indicator for iron status.42-45 One challenge, however, is interpreting serum ferritin lab results. Normal reference ranges can vary by lab, but serum ferritin levels from 12 to 200 ng/mL are often considered normal.46
Iron deficiency is likely when the ferritin level falls below 50 ng/mL. A large study of nearly 200 women aged 18 to 53 years confirms that iron supplementation should begin long before a person’s serum ferritin drops below 12 ng/mL. Women were admitted into the study if they had normal complete blood count (CBC) test results but levels of ferritin less than 50 ng/mL and if they also complained of fatigue. In those women, taking iron supplementation for 12 weeks improved their energy by 47.7% compared to 28.8% in the placebo group (difference –18.9%, 95% CI, –34.5 to –3.2; P=0.02).47 Another study reported benefit from taking iron when ferritin was less than 50 ng/mL in people with restless leg syndrome (RLS).48
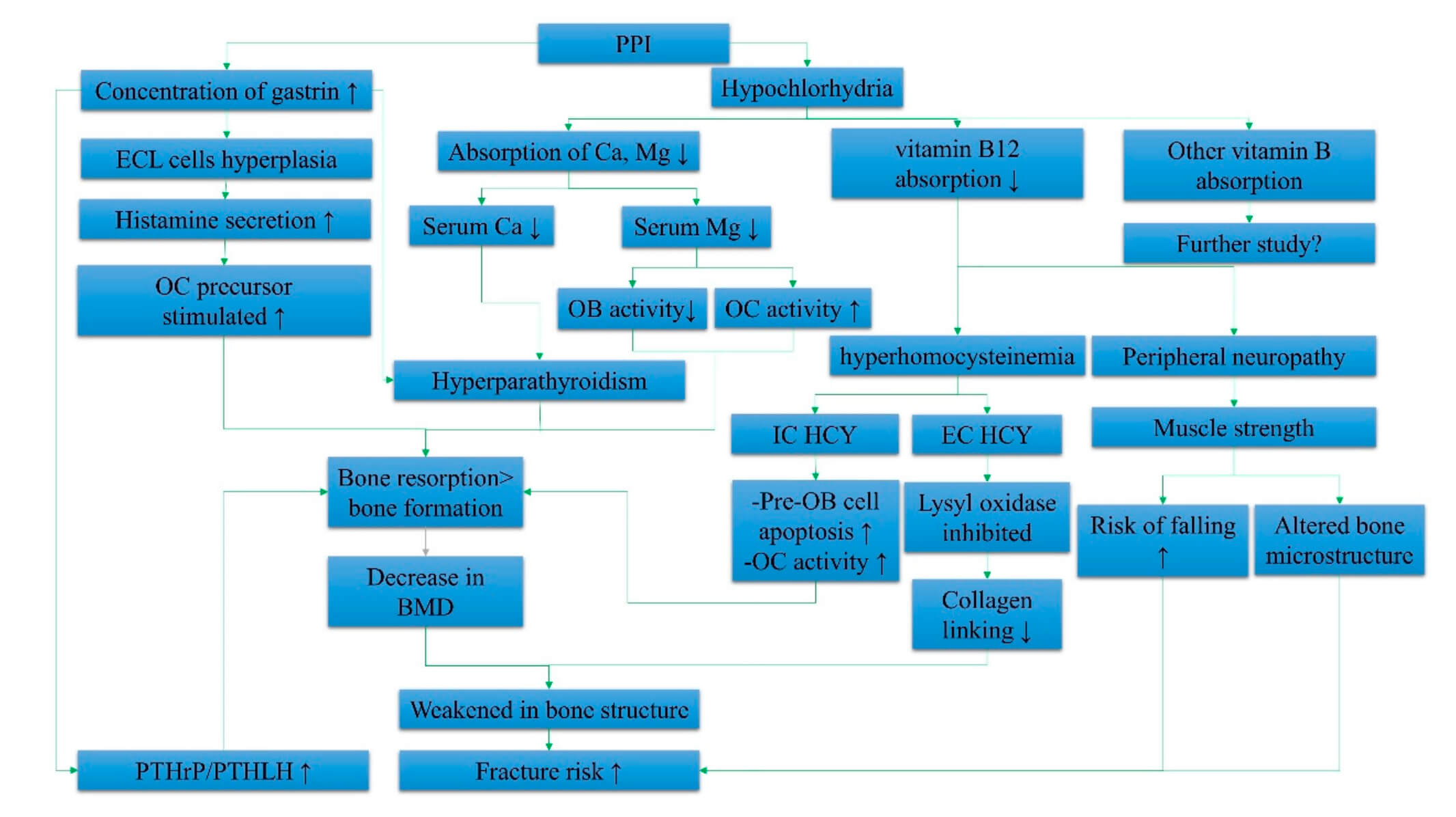
Figure 2. Summary of the systemic effects of PPIs in elevating fracture risk.39 Abbreviations: IC HCY, intracellular homocysteine; EC HCY, extracellular homocysteine; ECL cells, enterochromaffin-like cells; Ca, calcium; Mg, magnesium; BMD, bone mineral density; PTHrP, parathyroid hormone-related peptide; PTHLH, parathyroid hormone-like hormone; OB, osteoblast; OC, osteoclast; ↑ increase; ↓ decrease. © 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Antidepressants
Like acid-suppressive medications, antidepressants are 1 of the most prescribed classes of drugs. In 2018, 13.8% of all US adults and 24.3% of women aged 60 years or more took antidepressants.49 Even without adding a medication that can cause increased risk for osteoporosis and fragility fractures, those aged over 60 years are already the group at highest risk of osteopenia/osteoporosis.
Within the category of antidepressant medications, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are the most prescribed. The medications increase central and peripheral serotonin and are used to treat depression and anxiety disorders and other conditions, including premenstrual syndrome, chronic pain, fibromyalgia, peripheral neuropathy, and menopausal hot flushes. SSRIs include citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac), paroxetine (Paxil), and sertraline (Zoloft). SNRIs include atomoxetine (Strattera), desvenlafaxine (Pristiq), duloxetine (Cymbalta), and venlafaxine (Effexor XR).
A study published in the Archives of Internal Medicine obtained serial BMD measurements in a cohort of 2,722 older women (mean age, 78.5 years) participating in the Study of Osteoporotic Fractures, a prospective cohort study of community-dwelling women.50 Of those women, 198 were prescribed SSRIs and 118 took tricyclic antidepressant (TCA) medications. Over 4.9 years of monitoring, TCA use was not associated with a decrease in BMD compared to patients who did not take antidepressants.
That was not the case for SSRIs. On average, the women taking SSRIs experienced a higher age-adjusted rate of bone loss at the total hip than patients not taking antidepressants (−0.77% vs −0.49% per year; P=0.005). At any site, the rate of loss was 1.6 times higher in patients taking SSRIs compared to those not taking antidepressants. Interestingly, there was no significant difference in the rate of bone loss between women who reported at only 1 follow-up exam that they were taking SSRIs, called “partial users,” and those who reported taking the medications at 2 or more follow-up exams. The mean adjusted rate of total hip bone loss was −0.47% (95% CI: −0.54% to −0.42%) per year for nonusers), −0.83% (95% CI: −1.03% to −0.63%) per year for partial users, and −0.76% (95% CI: −1.14% to −0.38%) per year for continuous users.
A meta-analysis of 12 studies (7 case-controlled studies and 5 cohort studies) published in 2012 in the journal American Society for Bone and Mineral Research evaluated the association of SSRIs and fracture risk.51 The researchers concluded that SSRI use was associated with a significant increase in fractures compared to nonusers (AOR: 1.69; 95% CI: 1.51–1.90; I2=89.9%). In a subgroup analysis, they detected significant associations between SSRI use and hip or femoral fractures (AOR: 2.06; 95% CI: 1.84–2.30; I2=62.3%), spine (AOR: 1.34; 95% CI: 1.13–1.59; I2=48.5%), and wrist or forearm (AOR: 1.51; 95% CI: 1.26–1.82; I2=76.6%). Both high- and low-dose SSRI use was associated with significant increases in fracture risk. The risk for fractures was not significantly different between age groups or sexes. The authors estimate based on their study that clinicians can expect to see 1 fracture case for every 42 patients treated with SSRIs.
A second meta-analysis published in 2018 in the International Journal of Geriatric Psychiatry confirmed the earlier study.52 Compared to nonusers, the relative risk of fractures in patients taking SSRIs was 1.67 (95% CI: 1.56–1.79; P=0.000). Interestingly, the researchers found a significant duration impact on fracture association. Taking SSRIs for 1 year or less was associated with a 2.9% increase in fracture risk, or 1 additional fracture in every 85 users. Patients taking SSRIs for 1 to 5 years had a fracture risk of 13.4%, or 1 additional fracture in every 19 users.
In this meta-analysis there were not enough studies using SNRIs to determine the effects on bone of that category of medications. However, SNRIs were shown in a 10-year multicenter study in Canada to increase fracture incidence similarly to SSRIs.53 In this study, the HR for fracture in patients using either a SSRI or SNRI was 1.68 (95% CI: 1.32–2.15).
Mechanisms of Action
The proposed mechanisms of action by which SSRI and SNRI medications increase fracture risk are mediated by increased peripheral serotonin levels. More than 95% of serotonin is produced in the periphery, mainly by enterochromaffin cells,54 and more than 95% of blood serotonin is concentrated in platelets and does not cross the blood-brain barrier.55
Both centrally and peripherally acting serotonin affects bone (Figure 3). Central serotonin indirectly affects bone by decreasing sympathetic tone. The downstream effects of this are to increase osteoclast activity and reduce osteoblast activity, thereby stimulating bone loss. In contrast to central serotonin, peripheral serotonin acts directly on bone. Osteoblasts and osteoclasts contain serotonin receptors, neurotransmitters, and transporters.56,57 Gut-derived serotonin directly inhibits bone formation, decreasing osteoblast proliferation.55
Clinical Recommendations
There are no published guidelines on prevention or treatment of SSRI-induced bone loss. Clinicians should get a baseline and routinely order follow-up bone density tests. Given that MK4 is the only nutrient shown to attenuate drug-induced bone loss and also reduce fractures in postmenopausal osteoporosis, clinicians should consider supplementing with MK4 (45 mg/day), in addition to calcium and vitamin D.
Additionally, since underlying nutritional deficiencies are associated with depression, testing for these nutrients may help guide clinical decisions for creating targeted nutritional programs to help their patients. These include vitamins B3, B6, B12, and folic acid;58-60 iron (serum ferritin); magnesium;61 and the amino acids L-tryptophan, phenylalanine, tyrosine, and methionine.62-64 Functional testing of urinary organic acids, plasma amino acids, and red blood cell (RBC) minerals is one approach for determining nutritional insufficiencies. Treatment plans based on this approach have been shown to ameliorate depression65 and may provide the opportunity to reduce or discontinue the antidepressant medication.
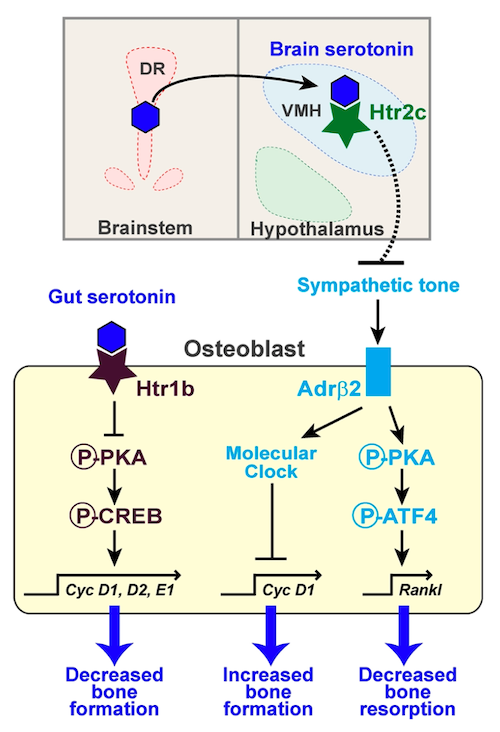
Figure 3. Gut- and brain-derived serotonin effects on bone.66 Gut-derived serotonin directly signals osteoblasts by binding to the 5-hydroxytryptamine receptor 1b (Htr1b). This inhibits cAMP response element binding protein (CREB) phosphorylation by protein kinase A (PKA), which reduces Cyclin (Cyc) gene expression, thereby decreasing osteoblast proliferation and slowing bone formation. In contrast, central serotonin works indirectly by inhibiting sympathetic tone. Dorsal raphe (DR) neurons release serotonin that signals to VMH neurons via the 5-hydroxytryptamine receptor 2c (Htr2c) to inhibit epinephrine synthesis and decrease sympathetic tone. This results in less β2 adrenergic receptor (Adrβ2) binding and inhibits osteoblast proliferation via a molecular clock gene/cyclinD1 (Cyc D1) cascade. This upregulates bone resorption by activating a PKA/ATF4-dependent pathway that increases receptor activator of nuclear factor kappa-Β ligand (RANKL) synthesis, which activates osteoclast differentiation and function. Sympathetic inhibition by brain-derived serotonin thus results in decreased bone formation and increased resorption. Solid lines, direct actions; broken lines, indirect mechanisms.
© 2010 Ducy and Karsenty. This article is distributed under the terms of an Attribution–Noncommercial–Share Alike–No Mirror Sites license for the first 6 months after the publication date (see http://www.rupress.org/terms). After 6 months it is available under a Creative Commons License (Attribution–Noncommercial–Share Alike 3.0 Unported license, as described at http://creativecommons.org/licenses/by-nc-sa/3.0/).
Aromatase Inhibitors
Aromatase inhibitors (AIs) reduce estrone and estradiol production and are first-line endocrine therapies for postmenopausal women with hormone-sensitive and metastatic breast cancer.67,68 The third-generation AIs are the most widely prescribed due to their high specificity for the aromatase enzyme and more favorable adverse-events profile compared with previous generations.69 These medications include exemestane (Aromasin), letrozole (Femara), and anastrozole (Arimidex).
Up to 80% of all breast cancer patients experience aromatase inhibitor bone loss (AIBL),70,71 and breast cancer patients hospitalized for a fracture have a higher risk of death compared with those without bone fracture (HR: 1.83; 95% CI: 1.50–2.22).72 AIs increase bone loss 2 to 4 times faster than natural, age-related ovarian failure, and women with breast cancer taking AIs have a fracture incidence of 18% to 20% after 5 years.1 This means that in clinical practice about 1 in 5 women on long-term AI therapy will experience an AI-related fracture.
[Aromatase inhibitors] increase bone loss 2 to 4 times faster than natural, age-related ovarian failure, and women with breast cancer taking AIs have a fracture incidence of 18% to 20% after 5 years. This means that in clinical practice about 1 in 5 women on long-term AI therapy will experience an AI-related fracture.
A Swedish study published in 2016 in the British Journal of Cancer Research evaluated medical records of 10,866 women with breast cancer (aged <75 years at the time of diagnosis) who were hospitalized due to bone fractures.72 The 5-year cumulative incidence of a fragility fracture for patients taking AIs was 5.9% (95% CI, 4.6%–7.6%), which, when compared to women without breast cancer, translated into a fracture incidence ratio of 1.32 (95% CI, 0.89–1.96). Compared to tamoxifen, AIs led to a greater increase in fracture risk.
The 5-year cumulative incidence of a fragility fracture for patients taking tamoxifen was 3.0% (95% CI, 2.3%–4.0%), but compared to women without breast cancer, there was a nonsignificant decrease in fracture incidence ratio of 0.94 (95% CI: 0.60–1.45). Postmenopausal women taking AIs were at higher risk for hospitalization with a fracture compared to those taking tamoxifen (HR: 1.52; 95% CI: 1.03–2.22). The results were similar when adjusted for age, tumor size, and lymph node status (HR: 1.48; 95% CI: 0.98–2.22).
Mechanisms of Action
AIs reduce peripheral estrogen levels below those attained from natural menopause, which causes accelerated bone loss and increased fracture risk.73-76 Low estrogen upregulates interleukin 7 (IL-7), which induces T-cell activation and a complex cascade of pathways that increase cytokine and reactive oxygen species (ROS), thereby increasing receptor activator of nuclear factor kappa-Β ligand (RANKL) and tumor necrosis factor alpha (TNFα).77 The net result is that estrogen deficiency upregulates osteoclast formation and increases the lifespan of osteoclasts, leading to increased bone loss, cortical porosity, and enlarged resorption areas.78
Clinical Recommendations
In 2017 a joint position statement on the management of AIBL was published in the Journal of Bone Oncology by the International Osteoporosis Foundation (IOF), Cancer and Bone Society (CABS), European Calcified Tissue Society (ECTS), International Expert Group for AIB (IEG), European Society for Clinical and Economics Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), International Menopause Society (IMS), and the International Society of Geriatric Oncology (SIOG).1 These organizations recommend denosumab or bisphosphonates for the prevention of AIBL, plus calcium and vitamin D.
Given that MK4 is the only nutrient shown to attenuate drug-induced bone loss and also reduce fractures in postmenopausal osteoporosis, clinicians should consider supplementing with MK4 (45 mg/day) in addition to calcium and vitamin D.
Androgen-Deprivation Therapy
Androgen-deprivation therapy (ADT) is used to treat polycystic ovary syndrome (PCOS), endometriosis, uterine myomas, breast cancer in premenopausal women, and prostate cancer. Androgen suppression is created through the use of gonadotropin-releasing hormone (GnRH) agonists, including leuprolide (Lupron, Eligard), goserelin (Zoladex), triptorelin (Trelstar), and histrelin (Vantras, Supprelin LA).
Treatment with a GnRH agonist in premenopausal women causes “clinical menopause”79 and increases the risk of bone loss and fractures.80 In premenopausal women taking a GnRH agonist, the bone loss has been estimated at 7.7% per year.81 However, significant bone loss has been detected much earlier. In premenopausal women treated with a GnRH agonist for endometriosis (14 women, aged 25–38 years) or uterine leiomyoma (4 women, aged 29–38 years), bone loss determined by a DEXA test occurred after 3 months of treatment and was statistically significant at 6 months (P<0.001).82
In men receiving ADT for prostate cancer, within 1 year of starting ADT absolute BMD loss is approximately 5%.83 Treatment duration increases osteoporosis risk, with 1 study that evaluated 390 prostate cancer patients concluding that osteoporosis incidence with ADT treatment was 42.9% after 2 years, 49.2% after 4 years, and 80.6% after 10 years.84
There is also a significant association with ADT use and fracture risk. A 2005 study published in the Journal of Clinical Oncology evaluated Medicare claims data from 3,887 men aged 65 years and older with diagnosed prostate cancer taking GnRH therapy compared to a control group of 7,774 men matched for age, race, and comorbidity (total cohort, N=11,661).85 The relative risk (RR) of any fracture in men taking GnRH therapy compared to the control group was 1.21 (95% CI: 1.14–1.29; P<0.001). For hip fractures, the RR was 1.30 (95% CI: 1.10–1.53; P=0.002). When controlled for metastases, which can increase fracture risk, the RR was similar for all fractures and hip fractures. Advancing age was an independent risk factor for fractures in men taking GnRH therapy. Compared to men aged 61 to 71 years, men aged 81 to 85 years and >85 years had higher hazard ratios for fractures, or 1.42 (95% CI: 1.28–1.59; P<0.0001) and 1.48 (95% CI: 1.32–1.67; P<0.0001), respectively.
Another study that evaluated data of 50,613 patients from the linked database of the Surveillance, Epidemiology, and End Results (SEER) Program and Medicare concluded that in men surviving for 5 years after diagnosis, 19.4% of those who received ADT experienced a fracture, compared to 12.6% of those not receiving ADT (P<0.001).86 Fracture risk increased with the number of treatments. Compared to men not receiving ADT, the RR of any fracture in men who received 1 to 4 doses was 1.07 (95% CI: 0.98–1.16); for 5 to 8 doses it was 1.22 (95% CI: 1.11–1.35); and for >9 doses it was 1.45 (95% CI: 1.36–1.56). The RR of fractures resulting in hospitalization was also associated with the number of doses, with a RR for 1 to 4 doses of 0.98 (95% CI: 0.82–1.17), for 5 to 8 doses of 1.51 (95% CI: 1.26–1.80), and for >9 doses of 1.66 (95% CI: 1.47–1.87).
Mechanisms of Action
GnRH therapy creates a hypogonadal state by inhibiting gonadotropins, thereby reducing testosterone and estrogens. The effect is chemical castration in men and a state resembling menopause in women. GnRH agonists create bone loss by increasing bone turnover and parathyroid hormone–induced osteoclast activity.87 Androgen deprivation causes an increase in interleukin-6 (IL-6), which stimulates osteoclastogenesis.88 Androgen receptors (ARs) on osteoblasts promote osteoblast differentiation, which decreases bone resorption.89 By reducing AR activity, the skeleton is deprived of its direct, bone-building effects. Similarly, by reducing estrogens, GnRH therapy increases osteoclastogenesis while reducing osteoclast apoptosis.90
Increased fracture risk with ADT is likely also due to osteosarcopenia. Decreased androgens lead to loss of lean muscle mass, decreased strength, and increased risk for falls and fractures.91 Men with osteosarcopenia have a fracture risk HR of 3.49 (95% CI: 1.76–6.90) compared to men with normal BMD and without sarcopenia.92
When men with osteosarcopenia break a hip, they’re also at higher risk for death. In a study of 324 patients (78 men, 246 women, mean age of 77.8+9.7 years), the 1-year mortality after a hip fracture in the men with osteosarcopenia was 25.8% compared to 2.1% in the men without osteosarcopenia (P=0.001).93 After adjusting for covariates, men with osteosarcopenia who fractured a hip had a 1.8 times higher mortality than nonosteosarcopenic men (HR: 1.84; 95% CI: 0.69–4.92). In women there was no difference in 1-year mortality after a hip fracture for those with osteosarcopenia compared to those without osteosarcopenia.
Clinical Recommendations
To counteract the risks associated with bone demineralization caused by GnRH therapy, treatment for endometriosis using a GnRH agonist should last only 6 months or less, followed by add-back hormone therapy.94 Clinical guidelines for managing ADT-induced bone loss in men undergoing prostate cancer treatment suggest assessing and monitoring bone density using a DEXA test, pharmacotherapy, exercise to promote strength and balance, calcium, and vitamin D.95
These guidelines are lacking in 2 respects. First, consuming adequate amounts of protein is crucial for preventing and reversing sarcopenia. The US recommended dietary allowance (RDA) for protein is 0.8 g/kg body weight per day for both men and women aged 18 years and more.96 However, 38% of adult men and 41% of adult women have dietary protein intakes below the RDA, and 15% of people aged more than 60 years consume less than 75% of the US RDA for protein.97,98
Research shows that consuming the minimum RDA for protein is not enough to maintain muscle mass in the elderly. In a 14-week study, volunteers received a controlled diet providing 0.8 g of protein per kg body weight per day and enough calories to maintain body weight.99 Volunteers were 4 men and 6 postmenopausal women aged 55 to 77 years (mean age of 66+3 years). After 12 weeks, mean mid-thigh muscle area, as measured by computed tomography (CT) scans, was reduced from 100.4+8.0 cm2 to 98.7+7.5 cm2 (P=0.019).
In contrast to the RDA, research suggests that a minimum protein intake of 1.0 to 1.3 g/kg body weight per day for the elderly, plus resistance training, can prevent and reverse the loss of muscle mass.100 And other recommendations go as high as 2.0 g/kg body weight per day.97
Secondly, clinicians should consider recommending MK4 based on the results of a 6-month, randomized clinical trial that showed taking 45 mg/day MK4 (alone and in combination with vitamin D3) attenuated bone loss in women being treated with a GnRH agonist.101 In this clinical trial, 110 women aged 24 to 52 years (mean 46.2+0.5 years) being treated for endometriosis and/or uterine leiomyomas were allocated into 1 of 4 groups. All women received subcutaneous leuprolide acetate, 1.88 mg/month. Group A received leuprolide alone; Group B received leuprolide plus MK4 (45 mg/day); Group C received leuprolide plus vitamin D3 0.5 mcg/day (200 IU/day); and Group D received leuprolide, MK4 (45 mg/day), and vitamin D3 (200 IU/day). Lumbar DEXA tests were done at baseline and at the end of the study.
In this study, adding MK4 or MK4 plus vitamin D3 to leuprolide therapy significantly attenuated bone loss. After 6 months of treatment, women taking leuprolide monotherapy (Group A) saw a mean lumbar BMD change of 5.25%+0.52%, while those receiving leuprolide plus MK4 (Group B) saw a mean BMD change of 3.72%+0.61% (P<0.05 for the difference between groups). Women taking leuprolide plus vitamin D3 (Group C) had a mean BMD change of 4.13%+0.70%. While the researchers did not report if the difference between the MK4 group (Group B) and MK4 plus vitamin D3 (Group D) was statistically significant, the greatest benefit was seen in patients taking leuprolide plus MK4 and vitamin D3 (Group D), with a mean BMD change of 3.59%+0.35% (P<0.01 for volunteers taking leuprolide plus MK4 and vitamin D3 compared to leuprolide monotherapy).
Conclusion
Medications are a commonly unrecognized cause of secondary osteoporosis. In an aging population, more people will be taking prescriptions and thereby increasing their risk for osteoporosis, fragility fractures, and death. Some of the most commonly prescribed medications negatively affect bone metabolism, cause osteoporosis, and increase fracture risk through direct and indirect mechanisms. Too often clinicians are not screening for osteoporosis in patients taking these medications, or they are putting them on osteoporosis-inducing medications even after the patient has sustained a fracture. Screening all medications for potential adverse skeletal events is crucial to ensuring patients are receiving the best and safest care possible.
Conflict of Interest Disclosure
The author of this commentary is the founder and President of Nutritional Biochemistry, Inc. (NBI), which manufactures bone health products containing MK4, calcium, and vitamin D3.
Abbreviations and Acronyms
Adrβ2: β2 adrenergic receptor
ADT: androgen-deprivation therapy
AI: aromatase inhibitor
AIBL: aromatase inhibitor-associated bone loss
AOR: adjusted odds ratio
AR: androgen receptor
ASCO: American Society for Clinical Oncology
ATF4: Activating transcription factor 4
BMD: bone mineral density
Ca: calcium
CABS: Cancer and Bone Society
CBC: Complete blood count
CDC: Centers for Disease Control and Prevention
CI: confidence interval
COPD: chronic obstructive pulmonary disease
CREB: cAMP response element binding protein
CT: computed tomography
Cyc D1: cyclinD1
Cyc: cyclin
DEXA: dual-energy X-ray absorptiometry
DR: Dorsal raphe
EC HCY: extracellular homocysteine
ECL cells: enterochromaffin-like cells
ECTS: European Calcified Tissue Society
ESCEO: European Society for Clinical and Economics Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases
FDA: Food and Drug Administration
GC: glucocorticoid
GERD: gastroesophageal reflux disorder
GH: growth hormone
GLOW: Global Longitudinal Study of Osteoporosis in Women
GnRH: gonadotropin-releasing hormone agonist
H2RA: histamine-2 receptor antagonist
HR: hazard ratio
Htr1b: 5-hydroxytryptamine receptor 1b
Htr2c: 5-hydroxytryptamine receptor 2c
IBD: inflammatory bowel disease
IC HCY: intracellular homocysteine
IEG: International Expert Group for AIB
IGA: immunoglobulin A
IGF-BPs: insulin-like growth factor bind proteins
IGF1: insulin-like growth factor 1
IL-6: interleukin-6
IL-7: interleukin-7
IMS: International Menopause Society
IOF: International Osteoporosis Foundation
Mg: magnesium
MIO: medication-induced osteoporosis
OB: osteoblast
OC: osteoclast
OR: odds ratio
PCOS: polycystic ovary syndrome
PKA: protein kinase A
PM/DM: polymyositis/dermatomyositis
PPI: proton pump inhibitors
PTHLH: parathyroid hormone-like hormone
PTHrP: parathyroid hormone-related peptide
RA: rheumatoid arthritis
RANKL: receptor activator of nuclear factor kappa-Β ligand
RBC: red blood cell
RDA: recommended dietary allowance
ROS: reactive oxygen species
RR: relative risk
RSL: restless leg syndrome
SEER: Surveillance, Epidemiology, and End Results
SIOG: International Society of Geriatric Oncology
SNRI: serotonin-norepinephrine reuptake inhibitor
SSRI: serotonin reuptake inhibitor
SLE: systemic lupus erythematosus
TCA: tricyclic antidepressant
TGFβ: transforming growth factor beta
TNFα: tumor necrosis factor alpha
URTI: upper respiratory tract infection
VMH: ventromedial hypothalamus






