Abstract
The principal purpose of this paper is to review various dosing options of naltrexone for use in clinical practice. Naltrexone can help treat a variety of conditions, including autoimmune diseases, neurodegenerative diseases, and acute or chronic pain, with the dosage affecting the results in a hormetic fashion. Practitioners historically have used naltrexone for the treatment of opioid addiction due to its opioid antagonistic properties.1 The Federal Drug Administration (FDA) approved the use of intravenous naltrexone in 1971, and thus, it became a common treatment for opioid overdose.2 The starting dosing of this medication for opioid addiction is a daily oral dose of 50 mg, while the maintenance dose may be higher.3 This dose is considered a “high dose” of naltrexone. For rapid response, the medication can be given intramuscularly or intravenously.4 For emergency treatment of opioid overdose, the general public can use intranasal naloxone. For most practitioners, the use of emergency interventions is not a part of regular practice. This article will contrast and review the use of naloxone and naltrexone in general clinical practice, including for intervening in situations of alcohol and opioid use and abuse.
The use of low-dose naltrexone (LDN) has been an established intervention in general practice for some years.5 The use of naltrexone in variable doses may be more valuable for providers in the daily treatment of diverse conditions, and can be very valuable when used in conditions such as alcohol abuse, inflammatory conditions, mood disorders, neurological conditions, and pain syndromes. This paper explores the use of naltrexone in high, low, very low, and ultra-low doses to assist the practitioner in making the most effective choice when using naltrexone in clinical practice. See Table 1.
Table 1: Naltrexone Dosing Options in Clinical Practice | ||
Type | Dose | Clinical Use |
High Dose | 50–200 mg | To reduce the need for higher doses of opioids and opioid abuse; alcohol use disorder |
Low Dose | 0.5–4.5 mg | To treat inflammatory and autoimmune disease, reduce pain, and improve fatigue and quality of life |
Very Low Dose | 0.001–1.0 mg | To manage opioid withdrawal symptoms and opioid detoxification |
Ultra-Low Dose | <0.001 mg | To help reduce side effects of opioids; synergistically used to decrease the need for higher doses of opioids; available intranasally |
What Is the Difference Between Naloxone and Naltrexone?
Naloxone
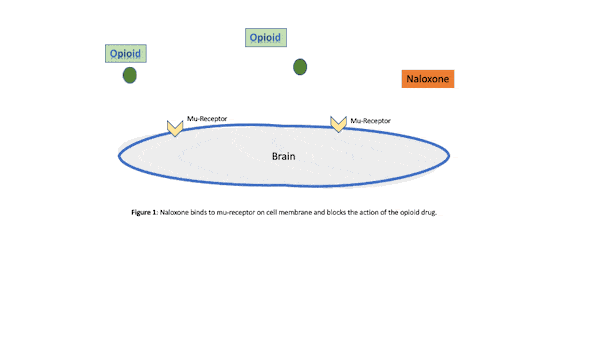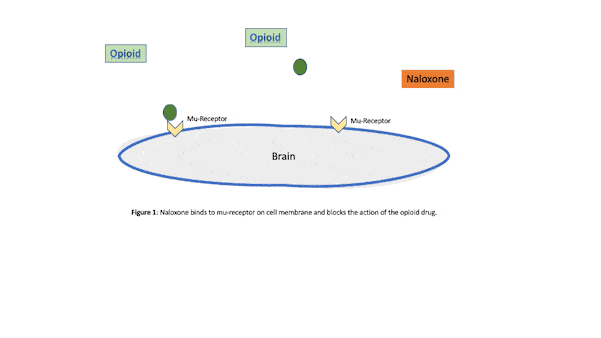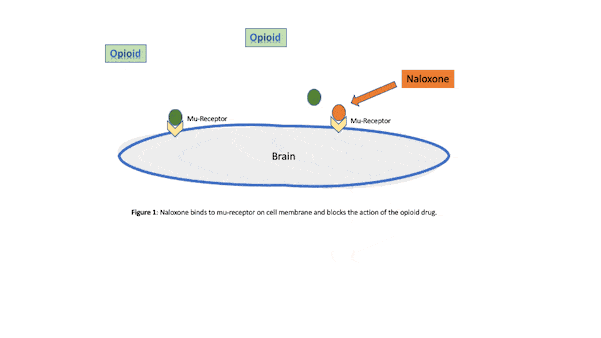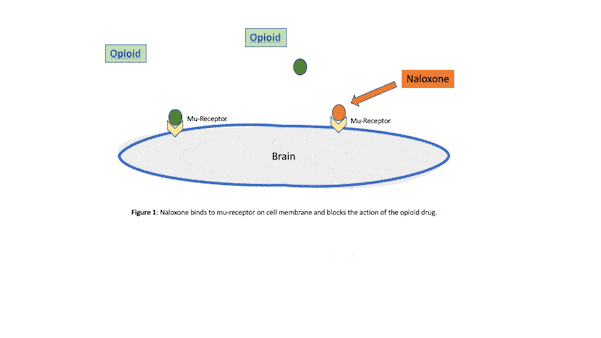
Naloxone is a short-acting medication used to reverse the effects of an opioid overdose and opioid use disorder.6 Naloxone is given for rapid reversal of opioid overdose and as a daily oral dose and long-acting injection prescribed to block both the pleasurable and sedative effects of an opioid drug like heroin or morphine (see Figure 1). Although intravenous administration offers the shortest onset for intervention of acute opioid toxicity, it can also be administered via nasal spray or subcutaneous or intramuscular methods.7
 Intramuscular injections of naloxone require less training; however, the onset of action is slower compared to intravenous therapy. The onset of action of intranasal naloxone is similar to intramuscular and requires minimal training for the general public. Opioid abuse is a major problem in the United States, so anything that supports the reduction of these drugs also supports a healthier people. It is critical that interventions available to the public be readily available and easily administered.
Intramuscular injections of naloxone require less training; however, the onset of action is slower compared to intravenous therapy. The onset of action of intranasal naloxone is similar to intramuscular and requires minimal training for the general public. Opioid abuse is a major problem in the United States, so anything that supports the reduction of these drugs also supports a healthier people. It is critical that interventions available to the public be readily available and easily administered.
One of the main characteristic symptoms of opioid overdose is respiratory depression, discernable by a respiratory rate of less than 12 breaths per minute for an adult. Intervention via intravenous naloxone can take effect within a few minutes, and the dose can depend on the amount of opioid in the system, as well as other variables. The initial dose of naloxone is 0.4 mg. Depending on response, this can be increased to as much as 15 mg.8 Intranasal naloxone requires about 5 minutes to take effect and offers an alternative for the general public due to its ease of use.9
The Surgeon General of the United States issued an advisory in 2018 encouraging the public to carry naloxone for opioid overdose. The following year, the FDA approved the first generic over-the-counter (OTC) intranasal naloxone.10 Concerns at that time included the need to have clear instructions regarding the proper use of naloxone to ensure the highest compliance possible. Pharmacies that offer intranasal naloxone without a prescription also provide specific instructions on the use of this medication. Additionally, the FDA publishes instructions for the public on the appropriate use of intranasal naloxone.11
Unlike doses used for opioid overdose, naloxone has been studied in lower doses for other purposes. The use of low-dose and ultra-low dose naloxone to reduce side effects of opioid agonists such as morphine has been studied, and researchers have reported some positive outcomes. In a study involving 60 postoperative patients, Fawzy et al reported that the use of low-dose, infused naloxone, when given with morphine sulfate, not only reduced opioid side effects, but also reduced the amount of morphine required for pain relief (P<0.001).12 Another study looking at the postoperative use of ultra-low dose naloxone on patients getting total abdominal hysterectomies found that 0.25 mcg/kg/h of naloxone for 24 hours not only reduced side effects of opioid medications, but also significantly reduced the amount of morphine used postoperatively (P<0.001).13
Although naloxone offers a life-saving intervention to opioid overdose and abuse, naltrexone offers a more practical use in general medicine due to its higher oral bioavailability. Dosing of naltrexone includes that which is considered to be high dose, low dose, very low dose, and ultra-low dose, sometimes referred to as pico- or micro-dosing depending on the source in literature.14
High-Dose Naltrexone
A very traditional approach to examining the use of naltrexone is to review the use of the highest doses of naltrexone. Naltrexone was developed in 1963, primarily for the purpose of treating alcohol and opioid dependence. The FDA approved the oral use of naltrexone in 1994 and the injectable form in 2006 for alcohol use disorder (AUD).15 The current standard starting dose of naltrexone for AUD is 50 mg daily in the oral form and 380 mg every 4 weeks in the intramuscular form. The therapeutic difference between naloxone and naltrexone is the onset of action, which makes naloxone more appropriate for opioid overdose. Oral naltrexone is also more orally bioavailable and has a longer duration of action as compared to naloxone. Oral naltrexone has about 6 times the absorption rate compared to naloxone, and the duration of action can last up to 3 days.16
Oral naltrexone in higher doses presents side effects. One of the main side effects of naltrexone in doses between 50 to 150 mg per day is nausea.17 Contraindications to the use of high-dose naltrexone include acute hepatitis or liver failure and current use of opioid drugs.18 In addition to side effects and contraindications, adherence to daily oral regimens is considered a major obstacle in the treatment of opioid use disorder (OUD). An implanted naltrexone device is currently being used in Europe and Australia; however, the FDA has not approved it for use in the United States.19
Low-Dose Naltrexone
In 1984, Bernard Bihari, MD, a Harvard University physician and researcher, observed that while naltrexone successfully blocked heroin’s ability to bind the opioid receptors, the complete blockage led to such severe side effects that the former addicts would not comply with continued use.20 With his knowledge of naltrexone’s effect on the immune system, he turned his research attention in that direction. Realizing that naltrexone’s effects included increasing endorphins and that this action raises immune competence, Bihari and others began testing innate endorphin levels in individuals with HIV/AIDS and found they were low in endorphin production compared to patients who did not have HIV/AIDS.21
The first clinical use of low-dose naltrexone (LDN) was in 1985 by Bihari. He compared various doses of LDN and found that 3.0 mg stimulated the body’s own production of endorphins but also the immune system.22 In an effort to find the minimal dose of naltrexone needed to raise endorphins, Bihari and his colleagues did a dose-ranging study comparing 50, 20, 10, 5, 3, and 1 mg of naltrexone.23 They found that while all these doses raised endorphins equally, a dose as low as 1.0 mg had no effect on endorphins. He then compared various doses of LDN between 1.75 and 4.5 mg and verified that a dose of 3.0 mg of LDN increased levels of endorphins during the night and even throughout the next day.24
Bihari stated in his research that endogenous endorphins were released in the body between 2:00 am and 4:00 am; however, other research has shown that beta (β)-endorphins are released in healthy adults between 4:00 am and 10:00 am.25 Most LDN is prescribed at bedtime, but if patients report nightmares, taking the dose in the morning is an alternative. Vivid dreams are the most commonly reported side effect in clinical trials, but this seems to decrease after a few nights. Another less common side effect is headaches, but these are reported as mild in severity. No side effects of stomach ulcers, renal impairment, or interference with anticlotting medications have been reported in research.26
Perhaps one of the most common uses of LDN is to reduce inflammation and manage autoimmune diseases.27 The increase of β-endorphins has been shown to help reduce pain levels, especially when the source of the pain is related to an inflammatory process. It has been shown that LDN reduces inflammation by reducing multiple pro-inflammatory cytokines.28 Cytokines are chemical messengers, often made by immune cells, whose net effect can be to either increase or decrease immune function. The coordination of the immune system rests on the body’s ability to keep a balance between cytokines that promote inflammation and those that reduce it.29 Tumor necrosis factor alpha (TNF-α) is also associated with both increased inflammation and neuroprotection when the body is presented with an insult such as nerve damage.30 Excess synthesis or upregulation of TNF-α is associated with certain conditions such as cerebral ischemia, Alzheimer disease, and atherosclerosis; reducing levels of TNF-α may be beneficial in treatment.31 There are well-documented studies on positive results when treating autoimmune conditions such as Crohn disease and rheumatoid arthritis.32
A more complex case involves individuals presenting with symptoms that involve both physical and emotional features; this gives the practitioner the opportunity to explore a monotherapy as an alternative to adding numerous pharmaceuticals and/or natural supplements. Fibromyalgia is a very common condition that fits this picture, and it also has been well-studied with LDN as a therapy.
Fibromyalgia is a generalized muscular pain syndrome. In addition to complaints of pain, patients with fibromyalgia also report symptoms of sleep disturbance and fatigue.33 In an 8-week, single-blinded pilot study involving 4.5 mg of LDN each night, serum levels of numerous proinflammatory cytokines, including interleukin 2 (IL-2) and TNF-α, were significantly reduced when compared to baseline in patients suffering from fibromyalgia (P<0.001).34 When practitioners recommend LDN for conditions as complex as fibromyalgia, it is important to work with the patient as a whole person, not just the pain (see Figure 3). Patients with fibromyalgia also have complaints of fatigue, depression, anxiety, and insomnia.35 Pharmaceutical treatment of fibromyalgia includes muscle relaxants, gabapentin, tricyclic antidepressants, selective serotonin reuptake inhibitors, and serotonin and norepinephrine reuptake inhibitors.36 These medications may be used in combination in an attempt to treat multiple concerns. The use of LDN as a monotherapy may offer the person suffering from fibromyalgia a solution for several concerns, including pain relief. In a pilot study using the Fibromyalgia Impact Questionnaire (FIQ),37 which measures improvement in pain, sleep, fatigue, and sadness, scores significantly improved over placebo when patients used a daily dose of 4.5 mg of LDN (P<0.0005).38
Figure 3: Treatment for patients with fibromyalgia should focus on the whole person.
Chronic fatigue syndrome (CFS), or myalgic encephalomyelitis (ME), is characterized by extreme and chronic fatigue. In addition, CFS patients often have myalgias, sleep disturbance, and cognitive deficits. A small study that compared healthy individuals with CFS patients found a significant improvement with impaired thought, concentration, and cognitive overload in the CFS group using 3.0 to 5.0 mg of daily LDN (P<0.004).39
The use of LDN to enhance quality of life has also been studied in multiple sclerosis (MS), an idiopathic inflammatory condition. Patients often complain of physical symptoms such as visual impairment, imbalance, weakness, and numbness. It is not uncommon for patients to also present with fatigue and depression. LDN may offer overall benefits for patients with MS, reducing both physical symptoms and improving the emotional and mental aspects of the condition. In a pilot study of 80 individuals with MS, those taking 4.5 mg of LDN daily for 8 weeks reported significant improvement in quality-of-life measures as measured by the Short Form-36 General Health Survey (P=0.01) and the Mental Health Survey (P<0.01).41
An open, uncontrolled study of 38 patients with primary progressive multiple sclerosis (PPMS) found a significant improvement in spasticity (P<0.01) following 6 months of treatment as evaluated by the Modified Ashworth Scale. Individuals in the study used 2.0 mg of LDN for the first 2 weeks, then 4.0 mg during the remaining 6 months. Also, individuals receiving the same dosing regimen were found to have a reduction in depression as evaluated by the use of the Beck Depression Inventory Scale (P=0.09). There was not a significant improvement in fatigue; however, there was an overall decrease in reported pain (P=0.01).41
The most common pharmacological interventions for MS include disease-modifying antirheumatic drugs (DMARDs) and immunosuppressive agents such as interferon beta, glatiramer, fingolimod, teriflunomide, dimethyl fumarate, natalizumab, and mitoxantrone.42 Practitioners may consider LDN for individuals who are unable or unwilling to take immunosuppressive medications or DMARDs, or for those choosing to use the LDN as adjunctive care. Researchers at Penn State Hershey conducted a 10-year retrospective study comparing the use of LDN alone with LDN plus glatiramer (Copaxone) on patients suffering from relapsing-remitting MS. For those individuals using only LDN, there were similar findings in both groups regarding walking times and changes on magnetic resonance imaging (MRI). These findings suggest that LDN may be considered a viable option for a long-term approach to conditions like MS. The authors note the need for patient trials regarding the use of LDN for patients with MS.43
Paradoxical Effects of Opioid Agonists and Antagonists
Treating patients with too low of a dose of opioid medications will not provide relief from pain and may result in hyperalgesia. Treating patients with higher doses on a chronic basis can lead to a tolerance to the inhibitory actions of opioids, thus resulting in an increased sensitivity to pain, opioid tolerance, and hyperalgesia (See Figure 4).
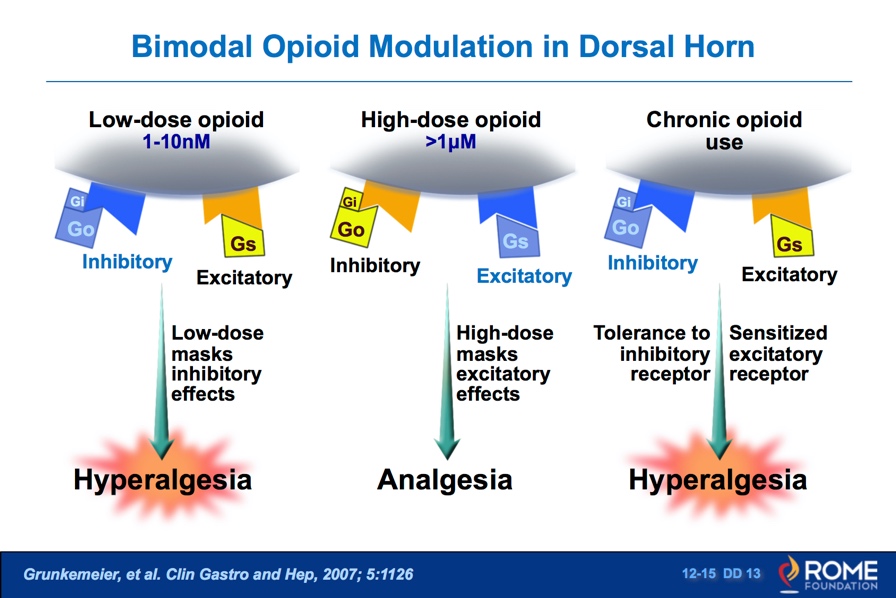
Figure 4: Using lower-than-effective doses of opioids can have similar hyperalgesia consequences as those in opioid-tolerant individuals. Used with permission from Rome Foundation.
Opioid agonists (eg, morphine) and opioid antagonists (eg, naltrexone) exhibit both excitatory and inhibitory action; however, these responses appear to be quite contrasting. Opioid agonists demonstrate predominantly excitatory responses at very low doses, while an inhibitory response occurs at high doses. Opioid antagonists, on the other hand, exhibit anti-excitatory responses at very low doses and anti-inhibitory responses at higher doses (see Figure 5).44
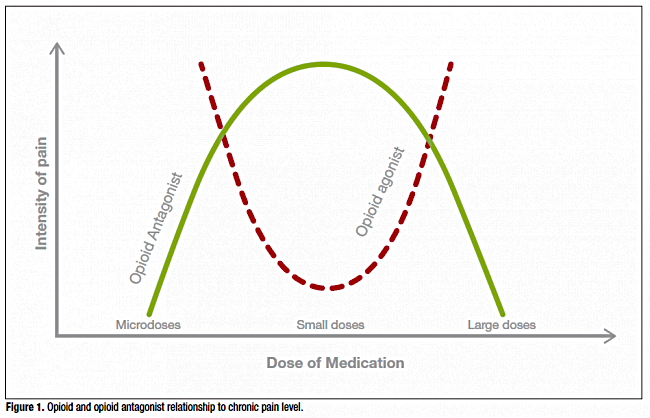
Figure 5: Opioid agonists demonstrate predominantly excitatory responses at very low doses, while an inhibitory response is noted at high doses. Opioid antagonists, on the other hand, exhibit antiexcitatory at low doses and anti-inhibitory at higher doses. Used with permission from Psych Scene Hub (https://psychscenehub.com).
Although researchers cannot fully explain the mechanism, the use of very low–dose naltrexone may assist patients with the opioid withdrawal process.
Very Low-Dose Naltrexone
One very interesting phenomenon in pharmacology is hormesis, which is similar to the bimodal theory. Basically, when we think of the drug naloxone or naltrexone, the phenomenon of hormesis refers to the stimulatory (excitatory) effects at low and very low doses and the inhibitory effects at higher doses.45
Very low–dose naltrexone (VLDN) has been used conventionally to aid in helping patients’ withdrawal symptoms when coming off opioid drugs. The dose associated with VLDN ranges from 0.001 to 1.0 mg. Higher doses of VLDN can reach the level of LDN, so there may be some overlapping benefits of both these approaches.46
Clonidine and lofexidine are alpha-2-adrenergic receptor agonists used off-label for opioid withdrawal.47 In a large study comparing daily dosing of 0.125 to 0.25 mg of VLDN with and without the use of clonidine, the VLDN group had a clinically significant reduction in restlessness, craving, and anxiety when compared to clonidine alone (P=0.02-0.001). The withdrawal-intensity scores for those receiving VLDN were also much better than for the clonidine group (P=0.03).48 Another study compared withdrawal symptoms from opioid medications using methadone with and without VLDN. Two groups received daily LDN, one using 0.125 mg and the other 0.25 mg. Researchers evaluated the groups using the Subjective Opiate Withdrawal Scale (SOWS), which reported the withdrawal symptoms of patients who were being tapered off opiates.49 The groups taking both doses of VLDN saw an improvement in SOWS scores as compared to placebo (P=0.001). The groups using VLDN also had a clinically significant reduction in cravings, especially during the last 4 days of the study compared to the placebo group (P=0.001).50 As practitioners primarily use VLDN to assist in reduction of withdrawal symptoms, ultra-low-dose naltrexone (ULDN) has been shown to both reduce the side effects of opioid medications51 and possibly to reduce the amount of opioid medication needed to initially treat patients to reduce pain levels.52
Ultra-Low-Dose Naltrexone
Opioid medications are well-known for potentially causing unwanted side effects, including nausea, vomiting, constipation, sedation, and respiratory depression.53 Opioids are also notorious for issues around tolerance and addiction.54 The use of ULDN can offer support for those requiring opioids while at the same time reducing possible side effects. One of the proposed mechanisms behind the side effects involves the excitatory pathways. ULDN can assist in reducing or blocking this mechanism in the gastrointestinal tract without interfering with opioids’ analgesic properties.55
According to the World Health Organization, palliative care is an approach that improves the quality of life of patients and their families facing the problems associated with a life-threatening illness, through the prevention and relief of suffering by means of early identification, impeccable assessment, and treatment of pain and other problems, physical, psychosocial, and spiritual.56 Palliative care is a good example of how ULDN may help to reduce secondary side effects such as nausea, vomiting, and constipation. One of the most common uses of opioids in palliative care is to help control pain, and up to 50% of patients receiving palliative care complain of pain. Providing relief with the use of opioids while coadministering LDN to avoid other complications is compatible with enhancement in patients’ quality of life. Although it was preclinical, a 2002 study found that 1.0 mcg (0.001 mg) of LDN reduced overall gastrointestinal side effects from both morphine and oxycodone (P<0.05-0.001), without any reduction in the analgesic benefits of the opioids (P<0.01).57
Researchers conducted trials using a combination ULDN (0.001 mg twice daily) along with oxycodone (brand name Oxytrex). Compared to the group using oxycodone alone without ULDN, the group receiving twice daily dosing of naltrexone with oxycodone had a significant reduction in pain levels (P<0.001). Unfortunately, phase III trials of this medication were suspended due to a high dropout rate.58
Summary
Investigators continue to research the use of naltrexone in medicine to assist practitioners in treating a variety of situations including autoimmune diseases, neurodegenerative diseases, and pain management. Adjustable dosing of naltrexone can serve as a valuable alternative to opioid medications and as an adjunctive measure to reduce the need for higher doses of opioids, to reduce or eliminate the side effects of these drugs, or to work synergistically to avoid opioid tolerance and chronic hyperalgesia.
One limitation in the use of very low–dose and ultra-low-dose naltrexone is finding a compounding pharmacy that can make such low doses. In a conversation with several compounding pharmacies, I found that the dose of 0.125 mg of naltrexone is readily available in capsule form. One compounding pharmacy in Colorado can offer a 0.1-mg scored tablet, which could be used as a single dose of 0.05 mg. Another pharmacy in Florida can make a liquid form of naltrexone at 0.01 mg per mL and capsules between 0.025 to 0.075 mg.
The use of very low–dose naltrexone to help patients reduce unwanted withdrawal symptoms from tapering off opioid medications may be a very good option for the general practitioner. Using ULDN to help patients reduce unwanted side effects of opioid drugs may be more challenging given the difficulty of finding resources for these lower doses, so practitioners have to use clinical judgment and make sure patients understand and consent to any use of this medication in these small doses. It is very important that general practitioners work interactively with specialists prescribing opioid medications and attempting to help patients eliminate unwanted side effects, or when there is a need or desire to taper off opioid drugs.






