This article is part of our February 2024 special issue. Download the full issue here.
Abstract
Introduction
Methylsulfonylmethane (MSM), an organosulfur compound, is gaining importance as an antioxidant and anti-inflammatory, as well as a source of sulfur for the body. Maintenance of hair strength and health requires a steady supply of sulfur, which oral supplementation with MSM can provide. A clinical study was designed to observe the efficacy of MSM as an oral supplement (OptiMSM® [Bergstrom Nutrition, Vancouver, Washington, USA]) for mitigating hair loss and improving hair thickness and density as well as overall appearance via subject perception and micrometric measurements of hair thickness.
Method
A total of 41 female and male subjects, aged between 19 and 60 years and presenting telogen effluvium hair loss, were recruited for the study. An area of 1.7 cm x 1.0 cm was marked on the head and shaved for the phototrichogram analysis. The hair fibers within the delimited area were cut and photographed. From an adjacent site, a minimum of 30 hair fibers were extracted for micrometric measurements. The measurements were repeated after 120 days of product use.
Results
Significant improvements of hair density, hair-density terminal, and hair diameter were observed after 120 days of OptiMSM use. Subject self-assessment determined that the product was effective in reducing hair loss as well as improving the strength/resistance and volume of hair. The subjects observed growth of new hairs and declared that their hair was more voluminous and less brittle after 45, 90, and 120 days of product use.
Conclusion
Based on the confines and conditions of this study, MSM supplementation was effective in reducing hair loss and improving hair diameter and thickness.
Introduction
Hair decorates the face and is a physical medium of social communication. It protects from trauma, insect penetration, and electromagnetic radiation and insulates against heat loss and heat gain.1,2 The scalp contains a high density of hair growth with an abundance of sebaceous glands.2 This forms a distinct microenvironment with significant differences from the rest of the skin. This environment is a rich habitat for microbes.3 There are no significant differences in the number of hair follicles between men and women or between different races.4 Hair appearance varies due to the type of hair originating from the follicle and individual hair-care practices.3,5
As a result of diet deficiencies and the aging process, hair changes color (graying) and hair production is reduced. In addition, structural properties of the hair fiber, such as diameter, curvature, elasticity, torsional rigidity, and lipid composition, also change, thus impacting the manageability and overall appearance of hair.5
Hair Structure
Hair is made up of 95% keratin, synthesized by the epidermal keratinocytes. It is a fibrous, helicoidal protein that forms part of the skin and all its appendages (body hair, nails, etc.). Keratin is insoluble in water, thus conferring impermeability and protection for the hair. There are 18 amino acids found in hair, including proline, threonine, leucine, arginine, cysteine, and methionine.6 Keratin is especially rich in cysteine, which is a sulfur-containing amino acid capable of forming disulfide bonds between molecules, thus adding strength and elasticity to the entire structure (Figure 1). The sulfur compounds impart burning-hair smell especially during styling or excessive sun exposure.6
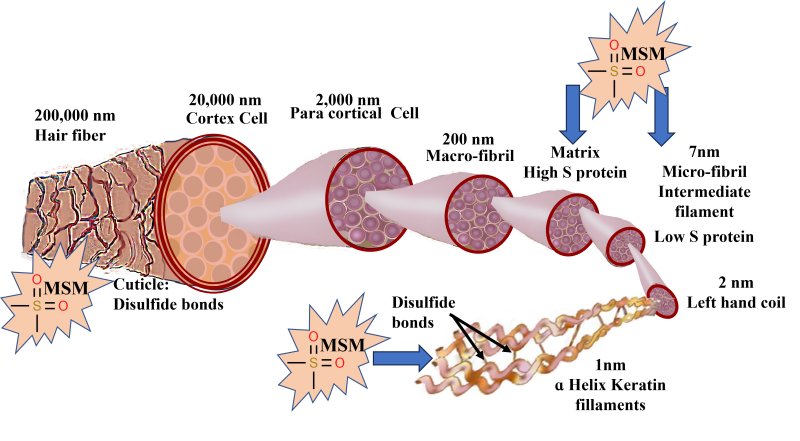
Figure 1: Schematic of hair fiber: The hair fiber is enclosed in the cuticle, a barrier protecting the underlying cortex. The cuticle comprises 8–10 layers of flat, overlapping cells stabilized and held in place by disulfide bonds. The cortex is in the center of each hair fiber and consists of long, tightly packed keratin spindles stabilized by disulfide bonds. These keratin spindles are arranged in a hierarchy, starting with the smallest structure, the keratin proteins, to the largest and final structure, the cortex. The keratin protein is a helicoidal group of cysteine-containing protein complexes with primary, secondary, and tertiary structures held together by peptide bonds, disulfide bonds, and hydroxide bonds. The disulfide bonds impart covalent bonding between adjacent protein chains and confer rigidity to the hair structure. Supplementation with OptiMSM provides an additional source of sulfur to strengthen the hair fiber.
Keratin-associated proteins are initially produced in the cytoplasm among keratin bundles that accumulate in cortical and cuticle cells of hair.7 When sulfur is readily available, keratin is made from strong disulfide bonds. When not enough sulfur is available, the body creates keratin with much weaker saline and hydrogen bonds. The primary source of sulfur is amino acids rich in this element, such as cysteine and methionine. The main source of these sulfur-containing amino acids is animal protein, which gives rise to important proteins, such as keratin, for hair-follicle growth.8
As a result of structural deformities and/or a reduction in the sulfur-containing amino acids cysteine and/or methionine, hair structure becomes fragile9-11 and vulnerable to excessive shedding. Ageing and excessive exposure of hair to solar irradiation cause dryness, roughness, and reduced strength and elasticity, which changes the hair appearance to a lackluster, stiff, brittle, and overall dull and unhealthy look.12 Severe degeneration of the cuticle and hair cortex has been observed in conditions of sulfur‐deficient hair (such as trichothiodystrophy).13 Sulfur contained in MSM could transfer to keratin and help strengthen the bonds between keratin molecules in hair (Figure 1).
Hair follicles go through cell cycles that include the following phases: anagenic, catagenic, and telogenic. The anagen phase is the actively growing phase during which hairs are produced at a rate of approximately 1 centimeter per month. In humans, the anagenic phase lasts 3 to 7 years. The catagenic phase starts after the anagen phase when the follicle no longer produces hair and starts to recede; it lasts approximately 10 days. Cellular division stops, and the follicle contracts toward the surface. The telogenic phase is a resting phase during which the hair strands shed. It lasts approximately 3 to 4 months until some unknown stimulus arouses the root, when it returns to the anagenetic phase. In a normal adult, approximately 85% to 95% of the follicles are in the anagenic phase, 1% to 2% are in the catagenic phase, and 10% to 14% are in the telogenic phase.14
Hair loss, known as telogen effluvium, occurs when the normal balance between growing and resting hair is altered and the telogen phase predominates. Several mechanisms can lead to telogen effluvium, but all involve the simultaneous entry of strands into the telogen phase.15 Telogen effluvium usually involves temporary, diffuse hair loss, while androgenetic alopecia is permanent and typically develops as a receding hairline or bald patch.
Oxidative metabolism, smoking, ultraviolet radiation, inflammation from microbial aggression, pollutants, irritants, and oxidized scalp lipids are some of the stress factors that affect hair fiber conditions before emergence of the hair from the scalp.16-18 Some oxidative stresses further impact emerged hair fiber; these include solar irradiation and chemical insults from oxidizing hair treatments such as colorants (Figure 1).19 Hair emerged from an unhealthy scalp has an altered cuticle with surface roughness, cuticle rigidity, and/or breakage. Rough cuticle surface appears as lackluster and dull-looking hair. Biochemical modifications, including alterations of protein and lipid components (mainly due to oxidative damage), also occur in hair emerged from an unhealthy scalp.20-23
Studies on reversal of hair loss indicate that the adult mammalian skin and hair follicle contain several distinct populations of stem cells that support substantial ability for regeneration. Hair follicle regeneration is contingent on the interaction between epithelial precursors located in the “bulge” and specialized mesenchymal cells found at the base of the follicle, called the dermal papilla.24-25 Dermal papilla cells provide instructive signals to induce epithelial bulge cell proliferation and consequent initiation of anagen (actively growing) follicle growth (Figure 1).26-27
Oxidative Stress and Hair
Oxidative stress is observed as an imbalance between the production of reactive oxygen species (ROS) and their elimination by protective mechanisms. Inflammatory pathways are triggered by oxidative stress, leading to subclinical, as well as chronic, inflammation. Oxidative stress is commonly detected in pathological conditions such as dandruff, seborrheic dermatitis, psoriasis, atopic dermatitis, telogen effluvium, and ultimately alopecia,28 and it plays a significant role in premature hair loss. The scalp commensal organism Melassezia spp has been recognized to be a source of oxidative damage.
Investigators have reported that oxidative stress can cause elevated myocardial ischemia markers in patients with telogen effluvium.29 Several studies have demonstrated that oxidative stress is associated with alopecia areata, and one showed an association between oxidative stress and androgenic alopecia.30-35 Oxidative stress causes lipid oxidation36 and lipid peroxides and may induce the apoptosis of hair follicle cells, followed by early onset of the catagen phase.23,37 MSM, as a known antioxidant, could quench these free radicals and reverse some of the oxidative damage (Figure 1).
Methylsulfonylmethane (MSM)
MSM occurs naturally in humans and animals, as well as some allium vegetables, green plants, and food items such as milk, grains, nuts, seeds, eggs, fruit, vegetables, turkey, beef, fish, and chicken.38-39 MSM is a source of sulfur for amino acids such as methionine and cysteine.40 Methionine is an essential amino acid, and as such, it cannot be synthesized in the body, so it has to be supplied by diet. On the other hand, cysteine is synthesized by the human body, but the process requires a steady supply of sulfur, which supplementation with MSM can provide.41-42 Pharmacokinetic studies indicate that orally administered MSM is rapidly absorbed in rats.38 In a human study, 45 subjects ingested 1, 2, or 3 grams of MSM daily for 16 weeks.44 Plasma MSM concentration correlated with the ingestion dose, indicating that MSM is rapidly absorbed in the upper gastrointestinal tract, slowly removed from the serum, and efficiently excreted from the body.43-44
Cysteine and methionine contribute significantly to the cellular pool of organic sulfur and general sulfur homeostasis, as well as carbon metabolism.45 Sulfur in these amino acids impacts the cellular redox state, thereby affecting the maintenance and integrity of the cellular systems, as well as the capacity to neutralize free radicals and reactive oxygen species.46 Quenching of free radicals reduces inflammation.46-47
Inflammation is a major manifestation of oxidative stress. Chronic inflammation, signified by the presence of lymphocytes and histiocytes, has been found in the tissue specimens of patients with hair loss.48 MSM has been reported to possess powerful anti-inflammatory properties. Studies show that MSM inhibits nuclear factor kappa-light-chain-enhancer of activate B cells (NF-kB), an intracellular protein complex involved in activating multiple inflammatory genes. In mechanistic studies done on rodents, MSM reduced the production of cytokines such as tumor necrosis factor alpha (TNF-ɑ) and interleukin 6 (IL-6), which are signaling proteins linked to systemic inflammation (Figure 2).49
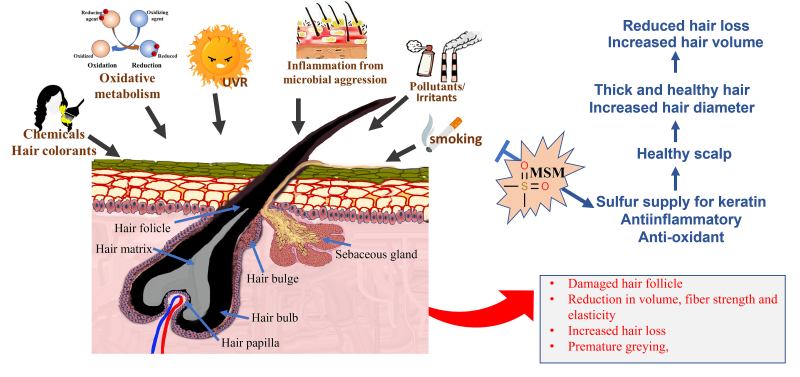
Figure 2: Various stress factors, including oxidative metabolism, smoking, inflammation from microbial aggression, environmental pollutants and irritants, ultraviolet radiation, and chemical insults from oxidizing hair colorants, cause damage to the scalp. The most common manifestation of hair emerging from an unhealthy scalp is an impaired hair follicle, weak and rigid hair fiber, and reduction in hair volume and hair loss. Supplementation with OptiMSM delivers anti-inflammatory and anti-oxidative resources, as well as an additional source of sulfur, which collectively heal the scalp, reduce hair loss, and aid in the emergence of thicker hair.
MSM is a “Generally Recognized As Safe” (GRAS)–approved substance. It is well-tolerated by most individuals at dosages of up to 4 grams daily, with few reported side effects,36 and has been reported to be nontoxic.38,50-51
Since MSM is a potent antioxidant and anti-inflammatory, as well as a source of sulfur for hair, it can be hypothesized that supplementation with this nutrient could improve hair condition. Previous studies indicate that oral supplementation with MSM influences skin and hair.52-54 This clinical study was designed to study the efficacy of oral supplementation with MSM on retarding hair loss and improving hair thickness, strength, and density, as well as overall appearance, under conditions of normal use.
Material
The subjects ingested 1 vegi-cap containing 1,000 mg of methylsulfonylmethane (OptiMSM®) orally for 120 days, with or without food intake.
Methods
This was a noncomparative clinical study conducted on the local population in Sau Paolo, Brazil. Female and male subjects, aged between 19 and 60 years (mean age: 43 years), presenting self-declared straight, wavy, curly, or frizzy hair of length longer than 10 cm (maintained during the study), were recruited for the study. The subjects were diagnosed by a dermatologist as having telogen effluvium hair loss at the inclusion. A total of 48 study subjects were recruited, out of which 41 completed the study (Figure 3). Six subjects discontinued due to product-unrelated reasons.

Figure 3: CONSORT flow diagram of subject recruitment and participation.
This study was conducted in compliance with the Declaration of Helsinki principles, the applicable regulatory requirements, including Resolution CNS no. 466/12, and according to the Good Clinical Practices, Document of the Americas, and ICH E6. This study was approved by the Independent Ethics Committee (IEC) of Investiga -Instituto de Pesquisas, registered by the National Research Ethics Commission (CONEP), Number 54859722.4.0000.5599, Opinion number: 5.263.427, 02/24/2022.
Subject Inclusion and Exclusion Criteria
The subjects were healthy males and females (aged 18 to 60 years) presenting telogen effluvium hair loss at the inclusion visit as assessed by a dermatologist. The subjects agreed to refrain from changing their hair dye routine for the course of the study. They were allowed to dye their hair 4-7 days before the visits (T0-D0, T45-D0, T90-D0, and T120-D0); however, they agreed to discontinue hair treatment products for hair loss, dandruff, supplements, etc for the course of the study. They also agreed to refrain from performing chemical treatments on the hair (smoothing, relaxing, progressive straightening, etc) as well as their hairstyle for the course of the study.
The subjects exhibited the ability to provide a consent for participating in the study and agreed to comply with the procedures, requirements, and schedules of the study. The subjects agreed to allow the shaving of an area of the scalp approximately 1.7 cm x 1.0 cm in size, as well as to the plucking of at least 30 hairs at visits T0-D0 and T120-D0.
Subjects diagnosed with cicatricial alopecia or pathological hair loss such as scalp disease (alopecia areata, universalis, or totalis) and those receiving chemotherapy were excluded from the study. The subjects did not suffer from type 1 diabetes mellitus, insulin-dependent diabetes, complications resulting from diabetes (retinopathy, nephropathy, neuropathy), dermatosis related to diabetes (lipoidica necrobiosis, plantar ulcer, ring granuloma, opportunistic infections), antecedents of episodes of hypoglycemia, diabetic ketoacidosis, hyperosmolar hyperglycemic syndrome, or skin diseases such as vitiligo, psoriasis, or atopic dermatitis. They were not on systemic corticosteroids or immunosuppressant treatment, and they were not pregnant or lactating. They did not report reaction to the category of product tested.
The subjects were excluded if they had moderate-to-intense dandruff or if they had more than 50% white hair and/or light-blonde hair color. The test site was devoid of moles, tattoos, scars, and any signs of skin irritation.
Methodology
On the initial visit (T0-D0), the subjects were informed of the study objective, methodology, and duration, and also about possible expected benefits and constraints related to the study. They were instructed to read and sign an informed consent form (ICF) and an informed consent for image release (ICIR).
An area sized 1.7 cm x 1.0 cm was marked and shaved on the top of the head for the phototrichogram analysis. Hair fibers, within the delimited area, were cut using fine curved tip scissors and shaved with a tricotomizer. Using the FotoFinder leviacam® device, a trained technician captured images of this area, which were then used for the analysis of the phototrichogram (scalp micrograph). Digital images were obtained for photographic documentation using a Canon EOS 60D digital still camera.
From a site adjacent to the shaved area, at least 30 hair fibers were plucked by a trained technician for hair micrometric measurements. The hair fibers were pulled 1 by 1 from the subject's head. The subjects returned to the institute 48 hours after the shaving (T0-D2), and a new image was acquired for phototrichogram analysis.
A similar 2-day procedure was repeated after using the supplement for 45 (T45-D0), 90 (T90-D0), and 120 (T120-D0) days. At each visit, the subjects were checked for compliance and possible signs of discomfort. Subjects completed a self-assessment questionnaire and had the same 1.7 cm x 1.0 cm area determined at the first visit shaved again. The subjects returned to the institute 48 hours after shaving (T45-D2, T90-D2, and T120-D2) and for additional images for phototrichogram analysis. At time point T120-D0, the subjects also underwent manual traction extraction of at least 30 hair fibers for micrometric measurements.
Dermatological Clinical Assessment
Study subjects were assessed by a dermatologist at the initial visit (T0-D0), to check the inclusion and exclusion criteria of the study. At all visits, the subjects were examined for possible adverse events or discomfort sensations and to confirm compliance with the instructions given to subjects at the start of the study.
Scalp Micrograph: Phototrichogram with FotoFinder leviacam®55
Phototrichogram (photographic trichogram) is used for in vivo study of the hair growth cycle. With the aid of a sanitized comb, a trained technician selected an area sized approximately 1.7 cm x 1.0 cm at the top of the head for shaving. The selected area was marked such that the FotoFinder leviacam® (Birnbach, Germany) captured the images from the same site at every visit. A measured area on the scalp was marked, and hairs were shaved within the target area. Photographs were obtained immediately after shaving (D0) and 48 hours after shaving (D2) with a digital closeup camera with epiluminescence microscopy for each evaluated time point. Tricholab software was used for image analysis. The software recognizes individual hair fibers in the photographs from D0 and D2 and measures the density of total hair fibers and density of hair fibers assigned as terminal hair (large and pigmented hair). By comparing the length of hair fibers in D0 and D2, the software can determine which hairs are growing and which are not.56-57 This method is employed for the study of hair growth rate, size of hair fibers, and frequency of telogen hair follicles and to quantify shed hair. In this project, the total hair density per square centimeter, as well as terminal hair density per square centimeter, was analyzed.
The phototrichogram analysis was performed at the following time points:
- T0-D0: Before OptiMSM intake.
- T0-D2: 48 hours after shaving at T0-D0.
- T45-D0: After 45 days of OptiMSM intake, before shaving.
- T45-D2: 48 hours after shaving at T45-D0.
- T90-D0: After 90 days of OptiMSM intake, before shaving.
- T90-D2: 48 hours after shaving at T90-D0.
- T120-D0: After 120 days of OptiMSM intake, before shaving.
- T120-D2: 48 hours after shaving at T120-D0.
Photograph for Documentation
Digital images of the head of all subjects (vertex) were obtained in a standardized configuration. Each subject’s hair was parted in the center. The subject’s head was then positioned on a Canfield Scientific hair photograph device (Canfield, New Jersey, USA) for full head/hair image capture, and a black cape was used to standardize color background. Digital images were obtained with a Canon EOS 60D (Canon, Tokyo, Japan) digital still camera, and medium standard views fixed lighting and vertex. Images of the entire head were taken with an aerial photo.
Images were captured at the following time points:
- T0-D0: Before OptiMSM intake.
- T45-D0: After 45 days of OptiMSM intake, before shaving.
- T90-D0: After 90 days of OptiMSM intake, before shaving.
- T120-D0: After 120 days of OptiMSM intake, before shaving.
Hair Diameter Assessment
The hair diameter (determined in the hair base) was measured using a Mitutoyo (Mitutoyo, Illinois, USA) micrometer (μm), a device capable of measuring the exact linear dimensions of an object, and the measurements were exported to the Instron® for analysis (Instron® Universal Testing System, Massachusetts, USA). At least 30 hair fibers were collected from each subject, including 25 hair fibers for measurements and 5 for replacement if necessary. The hairs were kept under controlled temperature and humidity before measurements. The Instron was used to measure hair diameter at the following time points:
- T0-D0: Before OptiMSM intake.
- T120-D0: After 120 days of OptiMSM intake, before shaving.
Self-Assessment Questionnaires Performed by the Study Subjects
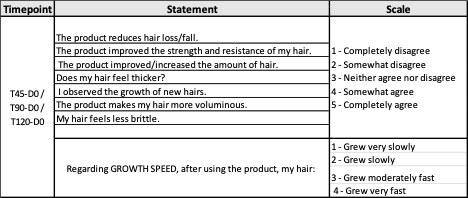
Final approval by consumers is what signifies the success of a product; thus, subject self-assessments were employed to provide a complete picture of product effect. The assessment by the subject was performed by following the “Standard Guide for Sensory Claim Substantiation” (ASTM E 1958-06, 2006), using a questionnaire for sensory studies to validate product claims. The study subjects were instructed to answer the following questionnaire:
Table 1: Time points, statements, and scale of the self-assessment questionnaires
Statistical Analysis
Excel statistical package was used for all statistical analysis. In addition to descriptive statistics, the Student’s T-test was applied to determine statistically significant differences from baseline at 95% confidence (two tailed).
Results
Phototrichogram Analysis
Hair micrograph density and terminal hair density count per cm2 are presented in Figure 4. A statistically significant (P<0.05) improvement of hair density was observed after 120 days of OptiMSM use. Hair density terminal also improved significantly (P<0.01) after 120 days of OptiMSM use. Figure 4b shows the number of subjects exhibiting improvement; 33%, 55.3%, and 66.7% of the subjects exhibited improvement in hair density after 45, 90, and 120 days, respectively. Terminal hair density per cm2 improved for 50% of the subjects after 45 days of MSM use, which increased to 57.9% and 82% after 90 and 120 days, respectively.
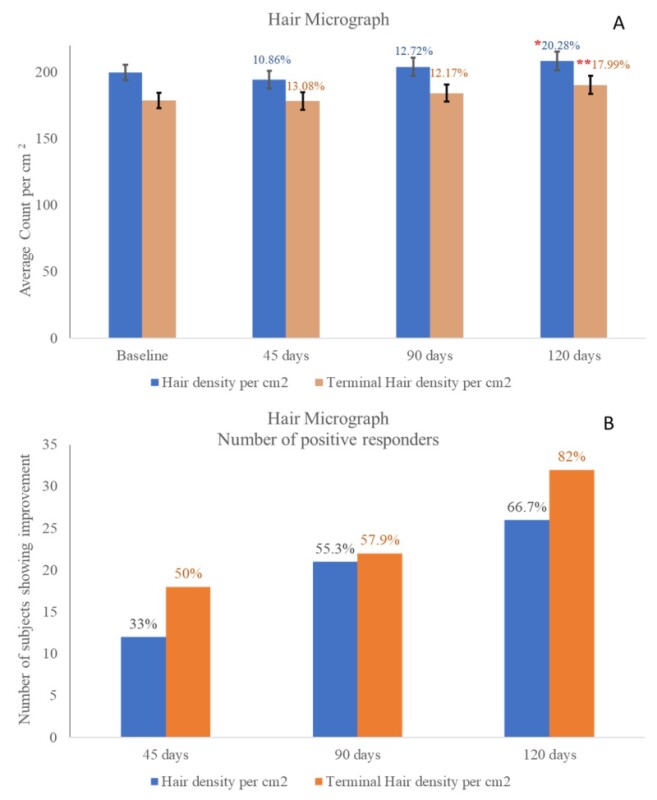
Figure 4: Figure 4a represents hair micrograph density and terminal hair density count per square centimeter. A single * indicates statistical significance with P<0.05, and a double ** indicates statistical significance with P<0.001. A statistically significant improvement in hair density was observed after 120 days of OptiMSM use. In addition, a statistically significant improvement of hair density terminal was observed after 120 days of OptiMSM use. Figure 4b shows the number of subjects exhibiting improvement.
Subject Self-Assessment
The subjects assessed their hair condition using a 5-point grading scale where a grade of 3 was neutral and above 3 indicated an improvement. When asked if they observed a reduction in hair loss, 77% of the population observed a reduction after 45 days, a percentage that improved to 82.9% after 120 days. Increased Strength and resistance of hair were observed by 85% of the population after 45 days of use and by 87.8% after 120 days (Figure 5).
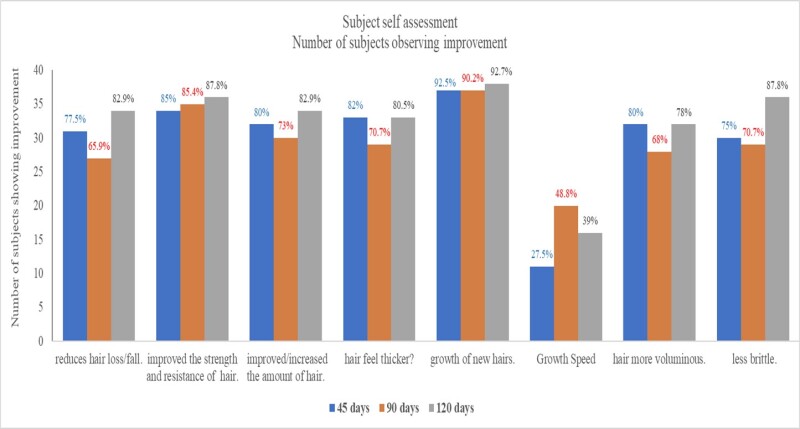
Figure 5: shows the number of subjects with scores of 4–5, which indicated improvement. Over 75% of the subjects observed a reduction in hair loss and improvement in strength and resistance of hair after 45 days, which increased further after 120 days. After using OptiMSM, over 80% of the subjects observed an increase/improvement of hair amount and hair thickness after 45 days, which increased slightly after 120 days. Growth of new hairs was observed by over 90% of the subjects after 45 days, and the effect was maintained for the course of the study. Speed of hair growth was not very noticeable to many subjects; however, 80% of the subjects noticed that their hair became more voluminous after using OptiMSM, which was maintained for the course of the study. Additionally, 75% of the subjects noticed their hair to be less brittle after using OptiMSM, which increased to 87.8% after 120 days of use.
After using OptiMSM for 45 days, 80% of the subjects observed an increase/improvement of hair amount and hair thickness, which increased slightly (82.9%) after 120 days. Growth of new hairs was observed by 92.5% of the subjects after 45 days, and the effect was maintained for the course of the study. Speed of hair was not very noticeable by many subjects; nevertheless, 27.5% of the participants did notice an improvement by 45 days, which increased to 39% after 120 days of use. Additionally, 80% of the subjects noticed that their hair became more voluminous after using OptiMSM, and this was maintained for the course of the study. Also after 45 days, 75% of the subjects noticed their hair to be less brittle after using OptiMSM, and this percentage increased to 87.8% after 120 days of use. Digital images of subjects’ heads (Figure 6) show a reduced appearance of the scalp.
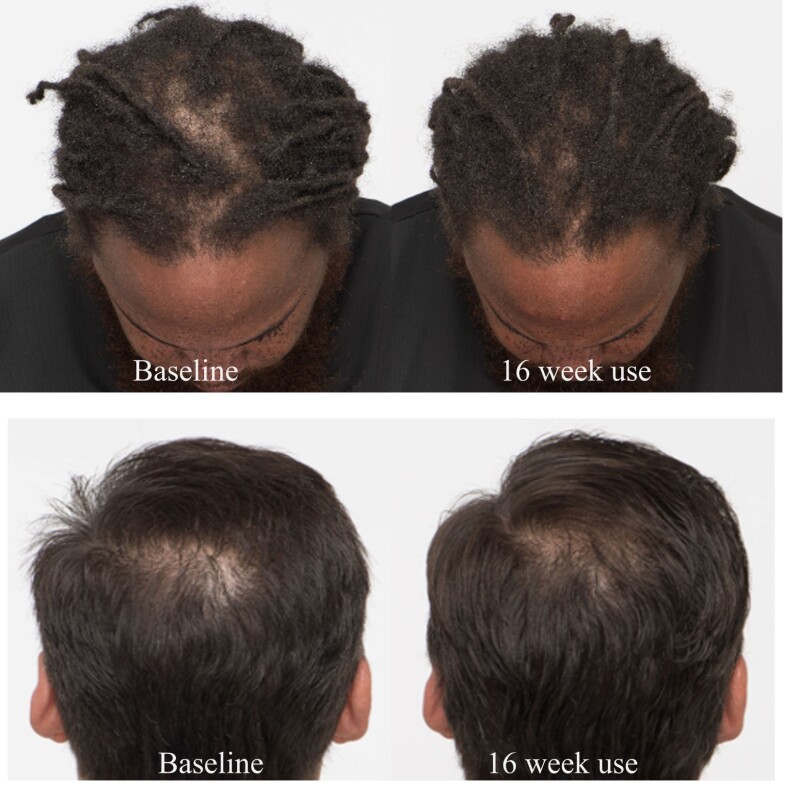
Figure 6: Photographs of the scalp before and after 16 weeks of treatment with OptiMSM. The scalp patches appear smaller after treatment.
Hair Diameter Assessment
The hair diameter measured at the base of the hair, using a micrometer, exhibited a significant (P<0.05) increase in hair diameter after 120 days of OptiMSM use (Figure 7). Wide hair diameter contributes to thicker, more-voluminous-looking hair.
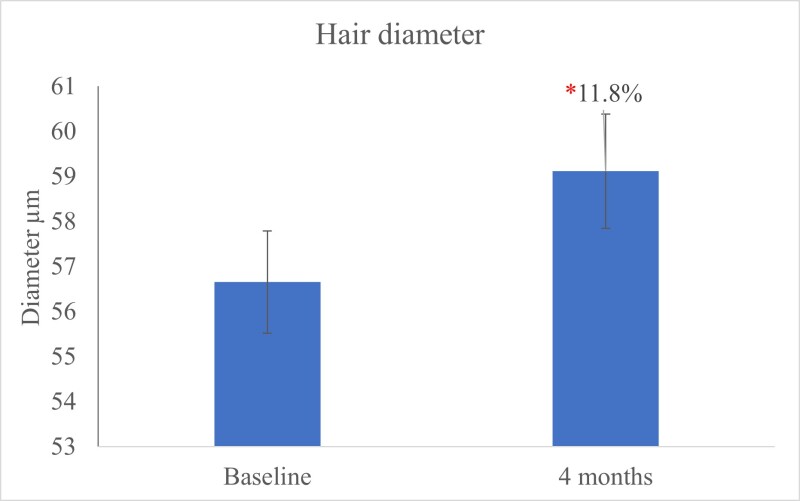
Figure 7: The hair diameter measured using a micrometer (μm) shows a significant (P<0.05) increase in hair diameter after 120 days of OptiMSM use.
Discussion
Human hair serves to protect the scalp, as well as contributing to the beauty, personality, and confidence of the consumer. External factors like exposure to the sun, smoking, dietary factors (including malnutrition of essential fatty acids and vitamins), and chemicals applied to the hair and scalp through shampoos and other treatments all can damage existing hair and impair hair growth. Age-related decline in keratin synthesis leads to deterioration of stability and flexibility of the hair shaft, leaving hair vulnerable to dryness and breakage with brushing and the use of styling products and tools. There is a myriad of topical hair treatment products professing to repair hair damage; however, nourishment of hair from within provides hair follicle cells the vital nutrients necessary to generate healthy hair. Oral supplementation with OptiMSM can benefit skin health because OptiMSM acts as a sulfur donor to keratin and also has antioxidant and anti-inflammatory properties.
Inflammation in the scalp and hair follicles can potentially contribute to dandruff, itchiness, and other conditions that can result in hair loss or stunt hair growth. By reducing inflammation, MSM may soothe the scalp and support healthy hair. In this study, 75% of the subjects noticed their hair to be less brittle after using OptiMSM, and this percentage increased to 87.8% after 120 days.
When sulfur is readily available, keratin is made from strong disulfide bonds.
Hair density refers to the number of hair strands growing per square centimeter of scalp, and a reduction in this permutation indicates hair loss. Hair loss can be reversed via the regeneration of stem cells present in the hair follicle. In this study, hair density and terminal hair density count increased significantly after oral supplementation with OptiMSM, indicating a reversal of hair loss. MSM is known to promote the formation of bonds at the follicle level. These bonds strengthen existing hair strands and help promote new growth, which was noticed by the 82% of participants who reported a reduction in hair loss after using OptiMSM in this study. Over 80% of the subject population also noticed increased/improved hair amount and hair thickness. Growth of new hairs was observed by 92.5% of the subjects, and 80% of the subjects declared their hair was more voluminous, indicating a significant role of OptiMSM supplement for providing nutritional benefits to hair.
The primary risk factor for baldness is the loss of hair thickness (diameter). This process starts in the hair follicle and, if untreated, leads to a decrease in the thickness of the hair shaft and, ultimately, hair loss. The hair diameter clinical scale via micrometer measurements indicates that the diameter of very thin hair is between 30 to 40 µm; medium hair is 50 to 80 µm; and thick hair is 90 to 110 µm.55 In this study, hair diameter increased significantly after using OptiMSM for 4 months. The sulfur in MSM is readily absorbed into amino acids such as creatine and methionine. This may encourage the formation of keratin and contribute to thicker hair. Improvement in hair strength and resistance was noticed by 87.8% of the subjects after using OptiMSM for 120 days.
While the results of this study are encouraging, additional studies are being designed to replicate these results, as well as to determine if this supplement could be useful for other causes of hair loss.
Funding
This research received no external funding. The study was fully funded by the sponsor, Balchem Corp.
Conflicts of Interest
RB is employed by Balchem, the supplier of the dietary supplement tested in this study.
JRC and LOG contributed to the execution of the study design and collection of data. They declare no conflict of interest.
NM conducted data analysis and prepared the manuscript and declares no conflict of interest.









