Abstract
Alpha lipoic acid (ALA), given orally or intravenously, affects many conditions where oxidation and inflammation are involved. One of the conditions for which exogenous ALA has been most studied is diabetes. In diabetes, oxidation leads to worsening hyperglycemia, and hyperglycemia leads to more oxidation, resulting in a cyclic pathophysiology. ALA is able to break this cycle by squelching oxidation, making it an attractive intervention in prediabetes and diabetes. There are at least 8 mechanisms to explain ALA’s beneficial effects for various conditions. Understanding ALA’s structure and use as an exogenous agent that is more akin to a drug than a nutrient, with particular attention to diabetes and diabetic peripheral neuropathy, is the topic of this review.
Introduction
Oral and intravenous ALA may benefit various conditions, including multiple sclerosis, cardiovascular disease, diabetes, obesity, and cognitive impairment.1,2 The data are particularly robust for its use in prediabetes (type 2) and diabetes (type 1 and 2) to mitigate comorbidities such as obesity, atherosclerosis, and cataracts.3-6 In addition, diabetic complications such as nonalcoholic steatohepatitis (NASH), retinopathy, and peripheral neuropathy may be reduced or thwarted altogether with ALA administration. A deeper understanding of ALA, with particular focus on insulin regulation and peripheral neuropathy, is the focus of this review.
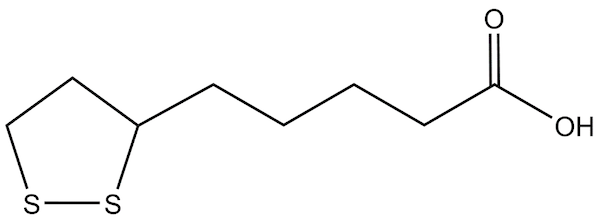
Alpha lipoic acid was first isolated from insoluble liver extracts in 1951 by Lester Reed and colleagues.7 This newly identified compound was named “lipoic” to mean lipid-like, due to its high solubility in organic solvents. The “alpha” designation was given because it was expected to be 1 of a series of similar compounds. (Beta lipoic acid exists, but it is not widely studied nor is it used as a drug or nutritional supplement.)8
Figure 1: Lipoic acid (oxidized)
In their seminal paper, Reed and colleagues showed that ALA could replace acetate in media used to grow lactic acid–producing bacteria, thus its early moniker as Acetate Replacement Factor. At the time, it was clear there was something in liver extract that could replace the otherwise requisite acetate in the media. Today, the fact that ALA may affect lactic acid–producing bacteria growth is intriguing given our growing understanding of the role these bacteria have in human health. The possible relevance of ALA to the microbiome is beyond the scope of this review, but future research into ALA’s effect on the microbiota may be informative.9-11
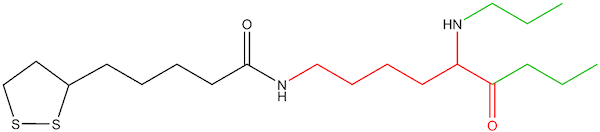
Naturally occurring lipoic acid (ie, the chemical structure as a free, unbound acid) is not part of the biology of humans or plants. Rather, cells biosynthesize a lipoyl moiety de novo, as an integral part of several multienzymes within the mitochondria (usually linked to enzymes via a lipoyl-lysine residue, Figures 2 and 3). The lipoyl-enzyme is confined within the mitochondria, with the lipoyl moiety acting as the catalytic site for the enzyme (the “lipoyl arm”).12 These enzymes take part in amino acid catabolism and energy production. Ultimately, these multienzymes have far-reaching effects on the viability of cells through nucleic acid synthesis and citric acid cycle kinetics, making the production of the lipoyl moiety essential to the viability of cells.13
Figure 2: Lipoyl-lysine (oxidized)
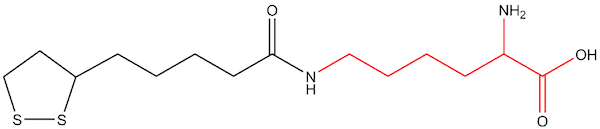
Figure 3: Lipoyl-lysine linked to enzyme
The activities of biosynthesized lipoyl-enzymes in the glycine cleavage system and in 4 alpha-ketoacid dehydrogenase enzymes within the mitochondria are well covered elsewhere.14 This review will focus on the role of oral and intravenous ALA as an exogenous source for clinical use.
Lipoic acid is often referred to as a “nutrient,” so one may assume that plants and animals absorb and use some lipoyl-enzymes in the diet. After all, plants and animals contain naturally occurring lipoyl-enzymes in their mitochondria. However, these lipoyl-enzyme complexes do not render any bioavailable ALA. This is suggested by data showing there is no measurable increase after meals.14 Normally, due to the confinement and usage of lipoyl-enzymes within the mitochondria, there is no measurable amount of ALA in circulation at any time. This means the only way to obtain measurable, circulating levels of ALA is through ingestion of a bolus dose, either orally or intravenously.
The lipoyl moiety is always part of an enzyme, so it is often referred to as “bound ALA” since it is covalently bound to a protein (an enzyme). In comparison, oral and intravenous ALA is not bound to any molecule, so it may be referred to as “free ALA.” In addition, there are 2 possible mirror images (enantiomers) of ALA: the R form and the S form. Naturally occurring lipoyl-enzymes are always synthesized in the R formation. Oral and intravenous ALA is available in either the R form or as a racemic mixture of 50/50 R and S isomers (alternatively called d and l).
Almost all of the clinical trials on ALA have used the racemic mixture (50/50 R and S enantiomers). Therefore, as a matter of convention, “ALA” generally refers to the racemic mixture. More recent studies may use the R form alone, which is designated as R-ALA or R-LA (R-lipoic acid).
Exogenous ALA, when taken orally or intravenously, is not an extraction like the original experiment done by Reed and colleagues in 1951. ALA is a synthetic molecule, no matter which conformation (R or S) or route of administration. The chemistry begins with cyclohexanone and vinyl ethyl ether and ends in the formation of ALA (Figure 4).
Figure 4: Chemical synthesis of ALA
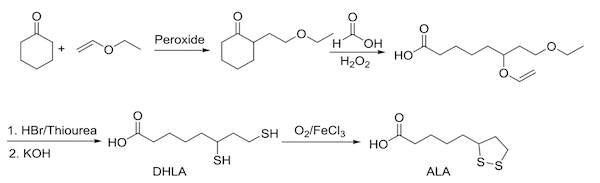
It appears unlikely that synthetic ALA offers a substantial means of substituting for the natural biosynthesis of the lipoyl arm of enzymes found in the mitochondria.15 While there is a transient salvage pathway that implies a minimal use within the mitochondria, it appears this is nearly immediate and cannot replace endogenous production. A good indication that synthetic ALA is not providing a meaningful amount of substrate for lipoyl-enzymes in the mitochondria is that those with rare inborn errors in genes necessary for endogenous ALA production do not derive therapeutic benefit from oral ALA.16
Of course, there are well-studied effects of synthetic ALA, some of which are outlined later in this paper. However, synthetic ALA should be regarded as more akin to a drug than a nutrient. Indeed, while ALA is available over the counter in the USA and Canada, in much of the world it is regulated as a drug. Intravenous (IV) ALA is a drug in all countries. In the USA, the Food and Drug Administration (FDA) undertook a review of IV ALA in 2018 and concluded that there are no current safety concerns and that the evidence is mostly supportive of IV ALA use in diabetic neuropathy, a use that Germany has approved since 1959.17
Biosynthesis of lipoyl-enzymes
As mentioned previously, plants and animals synthesize and use R-lipoyl-enzymes within the mitochondria. The synthesis of each enzyme’s lipoyl arm begins with the attachment of octanoic acid to a lysine residue of the enzyme, forming an octanoyl arm on the multienzyme. Then, 2 sulfanyl groups (-SH) are attached to the octanoyl moiety to form a dihydrolipoyl arm, and finally the dithiol group is formed through a dehydrogenase enzyme to give the lipoyl moiety of the multienzyme complex. Note that the lipoyl moiety is constructed after its fatty acid precursor is attached to the enzyme, rather than a simple attachment of a lipoic acid molecule to a protein. Indeed, there is no free lipoic acid made at any point in the biosynthesis pathway. Again, the entire biosynthesis of the enzymes themselves, with their lipoyl arms, takes place within the mitochondria, not the cytosol. There is also no transport, passive or active, out of the mitochondria of lipoyl-containing enzymes.
More recently researchers have discovered a salvage pathway that accounts for the uptake of lipoic acid into mitochondria.18 This is thought to be a minor contributor to the mitochondrial pool of lipoic acid and has been better characterized in prokaryotes to date.19 In short, the lipoate-activating enzyme acts on lipoic acid in an ATP-driven reaction. The intermediate is then acted upon by lipoyltransferase to render the end product a lipoyl-enzyme.20
Bioavailability of ALA as a Nutritional Supplement
Oral ALA is best taken on an empty stomach. Approximately 20% to 30% of oral ALA (free/unbound) is absorbed on an empty stomach when it is taken more than 30 minutes before a meal.21 When oral ALA is taken with food, peak plasma concentrations are reduced by 30% and total plasma concentrations by 20% versus taking it away from food.22 Both the R and the S isomers are rapidly metabolized and excreted, with peak concentrations occurring within an hour of ingestion.21,23 The excretion route is thought to be almost exclusively through urine.24
Of note, the R isomer is more bioavailable orally than the racemic mixture.25 In a small crossover study of 19 participants, there was better absorption of the R enantiomer, particularly in elderly participants (aged >75 years).26 However, this study used equivalent amounts of the 2 forms, 500 mg of ALA racemic mixture versus 500 mg of R-ALA. These results are only marginally relevant from a practical perspective, since R-ALA supplements are offered in doses much lower than racemic mixtures of ALA.
Once ingested, exogenous ALA transiently accumulates in the liver, heart, and skeletal muscles, although it can be found in various other tissues. Circulating metabolites include bisnorlipoate, tetranorlipoate, and beta-hydroxy-bisnorlipoate.2 These metabolites are in addition to ALA reduction to dihydrolipoic acid (DHLA), which is also rapidly excreted from cells.27 Once inside cells, ALA is extensively catabolized, mostly through beta-oxidation.28
Figure 5: Dihydrolipoic acid (reduced)
Mechanisms of Action of Supplemental (Free) ALA
- Direct antioxidant potential. Plasma-free ALA, which is exclusively gotten from oral ingestion or IV administration, is a potent reduction-oxidation (redox) molecule with both direct and indirect antioxidant action. Both ALA and its reduced form, dihydrolipoic acid (DHLA), are able to scavenge free radicals in cell culture. However, the relevance of direct free-radical scavenging in vivo is considered questionable.29 First, oral ALA leads to tissue concentrations that are 10 times lower than other well-known antioxidants (eg, vitamin C and glutathione), and secondly, in vitro studies show ALA is rapidly shuttled out of the intracellular compartment.26
- Regeneration of redox molecules. The role of ALA in regenerating other antioxidants leads to a potent net antioxidant effect. The action of scavenging free radicals necessitates that antioxidants be oxidized, rendering them incapable of accepting electrons again until they are reduced. ALA is a strong reducing agent for antioxidants such as vitamin C, vitamin E, and coenzyme Q10.27 This regeneration of potent antioxidants effectively recycles them so that they can repeatedly squelch free radicals (reactive oxygen species and reactive nitrogen species).
- Increase antioxidant enzymes. ALA is able to increase intracellular synthesis of glutathione (GSH) through the activation of Nrf2.30 Nrf2, or nuclear factor erythroid 2-related factor 2, is normally dormant in the cytosol in the absence of oxidative stress. ALA is able to activate Nrf2 by allowing it to be released from its “dock” (Keap1) and bind to the antioxidant response element on nuclear DNA, which promotes the expression of glutathione. In addition, ALA reduces the ratio of cystine to cysteine, leading to more availability of cysteine (the rate-limiting substrate for GSH synthesis).31
- Metal chelation. Free metals, including minerals such as copper and iron as well as toxic compounds such as arsenic and mercury, can damage tissue through oxidative mechanisms.32 ALA may mitigate the toxicity of metal ions on various tissue by directly chelating them, thus preventing their role as oxidants (eg, Fenton reaction).33
- Insulin sensitization/glucose uptake. Activation of 5’ adenosine monophosphate–activated protein kinase (AMPK) in muscle allows for improved entry of glucose.34 This may be mediated by upstream peroxisome proliferator–activated receptors (PPAR) alpha/gamma effects. For example, ALA has been shown to prevent hyperglycemic reduction of PPAR-gamma.
- Anti-Inflammatory. ALA inhibits the activation of nuclear factor kappa B (NF-kB) by preventing the degradation of its cytosolic dock, IkB (inhibitor of NF-kB). When NF-kB is docked to IkB in the cytosol, it is unable to traverse the nuclear envelope and bind to the promotor region of the many inflammatory genes.
- Inhibition of advanced glycation end-products (AGE). Protein glycation has been proposed as a mechanism of diabetic damage.35 ALA, as well as its oxidized form DHLA, can prevent AGE formation of proteins, including albumin. The prevention of glycation by ALA does not, however, affect plasma lipids.36
- Supports NO-mediated vasodilation. To the extent that reactive oxygen species (ROS) impair nitric oxide (NO)–mediated vasodilation, plasma-derived ALA antioxidant mechanisms (see No. 1 above) allow for mitigation of this effect. In addition, ALA restores endothelial nitric oxide synthase (eNOS) phosphorylation, leading to improved function of the enzyme and resulting in better nitric oxide–mediated vasodilation.37,38
Prediabetes/Diabetes—Relevant Pathophysiology
Oxidative stress, in the form of intracellular reactive oxygen and nitrogen species (ROS and RNS, respectively), is considered a causative factor in the development of type 2 diabetes.39 Conversely, hyperglycemia increases oxidative stress through several pathways, including
“a) stimulated polyol pathway where in ≤ 30% glucose can be diverted to sorbitol and fructose,
b) increased transcription of genes for proinflammatory cytokines and plasminogen activator inhibitor-1 (PAI-1)
c) activation of protein kinase-C (PKC) leading to several molecular changes
d) increased synthesis of Advanced Glycation End Products (AGEs)
e) changes in a receptor for AGEs and
f) auto-oxidation of glucose with formation of ketoimines and AGEs.”40
This vicious cycle of oxidation eventually results in diabetic complications, such as neuropathies, atherosclerosis, and cataracts.41 Disrupting this cycle with therapies that prevent formation of ROS and RNS may slow or even thwart the development of diabetes mellitus type 2 (DM2) and its complications.42-44
Insulin Sensitivity
The role of ALA as an insulin-sensitizing agent for cells has been recognized since the mid-1990s. In 1995, a trial of 13 patients with type 2 diabetes given a single infusion of ALA (1,000 mg) showed a 50% reduction in insulin-stimulated glucose disposal versus those given a placebo.45 Since then, many studies have corroborated the role of ALA as an insulin-sensitizing agent. A meta-analysis of 24 studies published in 2018 concluded that ALA improved glucose homeostasis as well as lipid profiles in subjects with metabolic syndrome.46
That said, ALA’s role as an adjunct to proper diet and lifestyle in prediabetics and diabetics (type 1 and 2) has amassed an impressive amount of evidence.
ALA is appropriate for prediabetics as well as diabetics, possibly forestalling or eliminating the progression to type 2 diabetes. High intake of fructose, particularly high-fructose corn or agave syrups, has been implicated in glucose dysregulation.47 ALA may lessen the risk of obesity and diabetes in those who consume standard Western diets, which are high in fructose.48
In a study of 74 patients with DM, participants were divided into groups receiving 600 mg of oral ALA once daily, twice daily, three times daily, or placebo. There was a significant increase in insulin-stimulated glucose disposal in those who received ALA versus the placebo, but it was not dose-dependent. This suggests 600 mg of oral ALA may be enough to improve insulin sensitivity.49
In a trial of adolescents with type 1 diabetes, lipoic acid (400 mg twice per day, lyophilized racemic mixture) alongside a high antioxidant diet (oxygen radical absorbance capacity [ORAC]=10,000/day; n=25) was compared to the same diet + placebo (n=27) as well as to a control group who had no diet instructions or lipoic acid (n=19).50 Endothelial function, which is expected to improve with ALA,51 was measured by reactive hyperemia peripheral artery tonometry technique. The high ORAC diet + lipoic acid group (n=25) had a significant improvement in their endothelial function versus both of the other groups. They also had a reduction in the amount of daily insulin needed, when compared to the other groups.
Diabetic Peripheral Neuropathy
Peripheral neuropathy is marked by sensorimotor symptoms such as numbness, tingling, lack of vibratory sense, and proprioception impairment; thus it can have grave consequences on the sufferer’s quality of life. Studies suggest that peripheral neuropathy affects prediabetics and diabetics at nearly equal rates, with incidence ranging from 30% to 50%.52,53 Oral ALA and intravenous ALA have been proven effective in reducing symptoms of diabetic peripheral neuropathy (DPN).
Several risk factors have been associated with a higher incidence of DPN, including advanced age, long duration of DM, poor control of hyperglycemia, increased lipid levels, high blood pressure, metabolic syndrome, genetic tendency, smoking, and low vitamin D levels.54-57 However, some people who develop DPN have none of these added risk factors. All diabetics should maintain foundational lifestyle habits that help stabilize glucose. In addition, oral or intravenous ALA may be added to prevent DPN as well as several other complications of DM.
ALA in intravenous form (600 mg/IV) given daily for 3 weeks is the best proven use of lipoic acid for relief of DPN.58 Several of the studies that used IV ALA had participants continue with oral dosing as part of their study design.
Oral ALA has had conflicting results on DPN. Some trials using doses as low as 600 mg/day have shown benefit, while other trials using doses as high as 1,800 mg daily failed to show benefit.34 Side effects of nausea and reflux are generally seen at high doses (1,800 mg/day). One trial comparing oral doses of 600 mg, 1,200 mg, and 1,800 mg daily found that all doses led to reduction in peripheral neuropathy symptoms. Since higher doses are more likely to lead to untoward effects and studies suggest subjective benefit in DPN at lower-end doses, a rational approach clinically is to begin with low-end dosing (600 mg/day) and then reassess symptomology.
Interestingly, there may not be only a dose but a duration of supplementation that affects the efficacy of ALA supplementation on DPN. A small study of 12 participants with DPN showed an improvement in both subjective and objective measures of neuropathy with a high-dose oral form that varied over time.59 In this trial, a single 1.6-gram tablet of R-ALA was given daily, and assessments of oxidation and DPN symptoms were done at baseline and days 15, 30, 60, and 120 of the study. Objective measures—motor-nerve conduction and sensory conduction velocity—improved at 30 and 60 days but deteriorated by day 120. Perception of wellness in participants was also greatly improved at day 30 but was not maintained over the course of the study. The authors noted that there may be a time variable, or even a “boomerang effect,” that should be accounted for in future studies of ALA.
It appears that subjective measures of neuropathy are improved with ALA even when objective measures of nerve conduction do not change. In a study of 32 consecutive DM patients with DPN, who were given 600 mg/day of ALA as a supplement, sural nerve conduction testing showed no change after 3 months.60 However, symptomology, as measured with the (NSS), did improve significantly over the same time period.
This is in keeping with a large multicenter trial, the NATHAN-1 cohort, that used subjective and neurophysiological measures to assess the use of 600 mg/day of oral ALA in 460 patients with DPN over 4 years.61 That study found significant improvements in the subjective scores of neuropathy without any change in the neurophysiological parameters assessed. The authors noted that ALA led to “clinically meaningful improvement and prevention of progression of neuropathic impairments and was well tolerated.”
Discussion
The primary role of ALA given in oral or IV form is as an antioxidant. In addition to scavenging free radicals, free ALA can regenerate other antioxidants intracellularly (eg, vitamin C, vitamin E, and glutathione) and upregulate intracellular antioxidant capacity through activation of Nrf2-signaling of the antioxidant response element. Prevention of oxidation may also be part of the therapeutic benefit of free ALA through the binding of highly redox-active metals (eg, iron) and inhibition of NADPH-mediated (nicotinamide adenine dinucleotide phosphate) production of superoxide free radicals.62
In addition to anti-inflammatory effects through reduction of oxidative stress, ALA has been found to have anti-inflammatory effects that are mediated through suppression of NF-kB activation.63 In a systematic review and meta-analysis of 18 trials on the use of ALA in people with metabolic syndrome, ALA significantly decreased C-reactive protein as well as tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6).64
Not all studies using lipoic acid have reported improvement in oxidative or inflammatory status. A study of 135 sedentary subjects with type 2 diabetes (average age=64, average body mass index [BMI]=28.5) found there was no change in measures of oxidation (superoxide dismutase [SOD]), glutathione peroxidase (GPx), or inflammation (CRP, TNF-α, IL-6, IL-8, and IL-10) after 6 months of oral ALA (600 mg/day of racemic ALA).65 There were also no significant changes in glycohemoglobin or 8-isoprostane levels.
This last study on sedentary subjects does suggest that lipoic acid should be considered as an adjunct to a diet that is already replete with antioxidant potential. The authors suggest future studies use higher dosing. A real-world scenario may be better represented by adding movement and a healthy diet to assess the addition of ALA to these foundational measures in sedentary subjects with diabetes.
Oral and intravenous ALA should not be considered a stand-alone treatment so much as an adjunct to other measures known to improve DPN. Studies have shown that symptoms of neuropathy may be partially ameliorated through lifestyle changes, such as increasing exercise, eating a high plant-based diet, and losing weight.66 Polyphenols, found in a high plant-based diet, may also ameliorate symptoms or forestall the onset of DPN through multitargeted interruption of cellular damage.67
It is possible that ALA has variable effects that depend on dose as well as duration of administration. In the study using 1.6 grams of R-ALA mentioned above, the authors noted a “boomerang effect” of peripheral neuropathy symptoms with continued use of an oral ALA, implying that over time there is a diminished efficacy. Several of the studies on DPN that showed benefit started out with IV ALA and had participants continue with oral ALA, suggesting this may be a practical way to address DPN. It is likely that we will need much more precise ways to measure the effects of ALA over time, both objectively and subjectively. Further clinical trials should be designed to use continual time points as well as observe biphasic effects of various dosages.59
Some precaution should be taken in using oral doses above 600 mg/day. First, ALA is a free acid and can lead to gastric upset in those who are inclined. In those who may have any swallowing difficulties, oral ALA is contraindicated due to the risk of acid damage to the esophagus. Toxic doses orally have been reported at 1,800 mg/day or more. Symptoms include nausea, vomiting, stomach pain, and fatigue. A recent case report of oral ingestion of 6,000 mg at once (10 pills, 600 mg each) led to delirium, metabolic acidosis, thrombocytopenia, and rhabdomyolysis.68
Preliminary data from animals have shown that at high concentrations oral ALA can lead to the destruction of islet cells in the pancreas. ALA should be carefully monitored with regular assessments of subjective and objective parameters of efficacy when used. As with any compound, it should also be used in the lowest dose possible to obtain the desired effect.
In 2014 Burton Berkson, MD, PhD, who has pioneered the clinical use of IV and oral ALA for many decades, authored a short article meant to remind practitioners that the safety and toxicity of IV ALA are known.69 In this article, Berkson refers to data from rhesus monkeys that determined that the LD50 toxicity dose is 90 to 100 mg/kg. To be clear, LD50 means that 50% of the monkeys died at that dose. All of the monkeys had acute hepatic necrosis with extensive damage to the mitochondria upon microscopic examination. Berkson goes on to write that he does not exceed 10 mg/kg in his practice with patients, and often the dose of IV ALA is much lower than that. A commonly used dose in practice is 600 mg over 45 minutes, which would equate to Berkson’s upper-level dose in a 60-kg (132-lb) person.
Conclusion
Alpha lipoic acid (ALA), as an oral or intravenous agent, should be thought of as a drug more than a nutrient. There is substantial evidence that ALA is appropriate for nearly all diseases of aging, from heart disease to dementia.70 That said, ALA’s role as an adjunct to proper diet and lifestyle in prediabetics and diabetics (type 1 and 2) has amassed an impressive amount of evidence. Intravenous use of ALA for peripheral neuropathy has enough evidence for its approved use in Germany. Oral supplementation has also provided symptomatic relief for those suffering from diabetic peripheral neuropathy. Optimal dosing and duration of ALA have not been definitively determined and may follow a nonlinear dose-duration relationship. The lowest dose shown to lessen symptoms of DPN is 600 mg/day, and the longest study duration has been 4 years at this dose.61






