Abstract
Small intestinal bacterial overgrowth syndrome (SIBOS) is a common problem for patients with irritable bowel syndrome. SIBOS results from opportunistic pathogenic bacteria colonizing in the small intestine where digestion and absorption primarily occur. SIBOS can cause many different ailments such as malnutrition, diarrhea, and bloating. Because of the location of the bacteria, the symptoms are usually at their worst directly after a meal when the nutrients that would normally be broken down and absorbed by the body are instead trapped and used by the offending microorganism(s). The purpose of this trial was to determine if a combination of naturally occurring antimicrobials can allow reduction and suppression of the invading microorganisms under physiological conditions, so when the combination is given to a patient before eating, the nutrients will be broken down and used by the body, the body will have time to heal, and the probiotics will establish control over the pathogenic bacteria.
Introduction
In healthy individuals, the proximal small intestine is relatively sterile except for a few colonies of Lactobacilli and other aerobic species that are regarded as beneficial bacteria.1 Greater numbers of microorganisms are found in the distal ileum. Significantly more microorganisms are found in the large intestine (>106 per mL) and include both aerobic and anaerobic species.2 The microorganism content of the gut has been shown to play a vital role in health.3,4 When these colonic microorganisms, as well as pathogenic bacteria, increase to >105 per mL and progressively invade and conquer the local small intestinal environment, a number of gastrointestinal (GI) symptoms present themselves, which is known as small intestinal bacterial overgrowth syndrome (SIBOS).5
The following factors contribute to keeping the duodenum and jejunum free of colonic or pathogenic bacteria: gastric acid secretion, normal intestinal motility, adequate concentrations of pancreatic enzymes and bile salts, intestinal immunoglobulins, and an intact ileocecal valve. When one or more of these factors is absent or altered, there is a concomitant increase in qualitative and quantitative growth of colonic and other microorganisms in the proximal small bowel. The most common species invading the small intestine in SIBOS are Escherichia coli and Bacteroides.6
Patients with SIBOS usually present with complaints of abdominal discomfort and pain, bloating and distention, intestinal gas, flatulence, and diarrhea. The offending microorganisms may alter the structure and function of the small intestine, which can lead to malabsorption syndrome. When these bacteria digest food in the small intestine, gas may be formed, as well as other irritating substances that cause diarrheal symptoms. The use of nutrients by the bacteria may also contribute to weight loss and nutritional deficiency states, not infrequently noted in patients with SIBOS.7
SIBOS occurs as a complication in many GI conditions. Irritable bowel syndrome (IBS), which occurs in more than 20% of the population, presents with a constellation of functional GI complaints. IBS is a symptom complex of unknown etiology that is mainly diagnosed when other GI pathologies are excluded. More recently, it has become evident that more than 50% of patients with IBS also have SIBOS.8 Thus SIBOS is prevalent in many individuals with functional GI complaints.1
Although the treatment of IBS is directed at ameliorating abdominal complaints, the management of SIBOS requires correcting the initial pathology and reducing the abnormal bacteria growing in the proximal bowel. Antibiotics have been used in repetitive courses to help reduce or eliminate the microbial flora and control many of the symptoms present in patients with SIBOS.9-11
The ideal treatment for people with SIBOS is to reduce the bacterial population in the small intestine and inhibit growth for 3 to 4 hours while food is broken down and absorbed in the small intestine. Many natural food components have been shown to be bactericidal or bacteriostatic to the growth of these pathogenic organisms. A number of natural compounds including enzymes, essential oils, peptides, and chelators possess antimicrobial properties and are safe for human consumption.12,13 The goal is to find the right combination of antimicrobials that can reduce and suppress bacterial growth under physiological conditions.
The aims of the study were to test the efficacy of these various natural ingredients alone or in combination for their properties of reducing the numbers and/or inhibiting the growth of the 2 most common offending bacterial species, namely E coli and Bacteroides. It is important to establish a good bacterial population while suppressing and reducing the bad bacterial flora. Therefore, a probiotic screen was conducted to determine which strains were capable with the antimicrobial cocktail because spores will be the main focus for the probiotic. For that reason, a number of Bacillus strains were tested for their compatibility in such a composition.
Materials and Methods
The objectives of the study were to perform in vitro culture studies to determine the efficacy of multicomponent enzymes and antimicrobial ingredients in the reduction and suppression of the growth of various SIBOS-causing bacteria. The enzymes, botanicals, probiotics, and/or chelators selected were based on literature reviews and technical reports that tested positive for antimicrobial properties. From those products, the list was narrowed to include only products available in the supplement industry that are denoted as “generally recognized as safe.” All of these studies were performed under low oxygen, at pH7.0, and at 37°C unless stated otherwise.
The bacteria selected for testing included Bacteroides fragilis and the Bacteroides uniformis (purchased from Sigma Aldrich, St Louis, Missouri) and 2 species of E coli, strains of E coli B and E coli C (a gift from Texas A & M University, College Station). The Bacteroides species were cultured under anaerobic conditions. The bacterial cells were activated on a nutrient agar plate at 37°C for 24 to 72 hours depending on strain. Cells of several colonies were incubated in 50 mL of nutrient broth until the culture reached an optical density of 0.2. Test substances were slowly added. Samples were taken at various points, plated on selective media for colony counts given in colony-forming units, and the optical density of culture was obtained at 600 nm. The optical density of the ingredients was subtracted out using a simulated culture with no inoculants.
The initial studies were performed on an individual basis to assess each ingredient’s antimicrobial activity. Amounts were used based on physiological conditions,14-16 and 2 to 3 different dosages were tested. Using these data, ingredients were selected based on how well they reduced the bacterial population along with how long they inhibited growth to create a composite blend. Each component of the antimicrobial combination was tested individually, as well as the combination as a whole, to confirm that all individual characteristics were maintained once combined. This antimicrobial cocktail resulted in a reduction by 2 to 3 logs of bacterial counts while inhibiting bacterial growth for 3 to 4 hours. Another set of experiments included the testing of the composite blend with various strains of Bacillus to determine survival of the probiotic while the composite blend inhibited the growth of the offending bacteria in our test system.
Antimicrobials were screened using growth curves and the logarithm of the relative population size [y=ln(NIN0)] against time. Frozen permanents of each strain were streaked for single colonies using nutrient agar plates warmed prior to use. Single bacterial cells were restreaked on a nutrient agar plate, and test ingredients were slowly added. The timer was started when the test substance was added to the reaction; addition of samples took less than 30 seconds. Samples were taken at various timepoints, and the optical density of the culture was obtained at 600 nm. All experiments were done in duplicate.
Survival Plating
Enumeration of tester strains under antimicrobial conditions was done by taking samples 6 hours after the addition of an ingredient from the experiments above and diluting them at several different concentrations using a phosphate buffer. Dilutions were plated on selective media and incubated at optimal growth conditions for 24 to 72 hours depending on strain.
Results
Antimicrobial Screening
Materials tested were chosen from the following 4 categories of compounds that are known to possess antimicrobial properties.
- Chelators. The outer membrane of gram-negative bacteria acts as a permeability barrier for the cell. It is responsible for preventing molecules such as antimicrobials, detergents, and dyes from reaching the cytoplasmic membrane.17 Magnesium ions serve to stabilize the lipopolysaccharide layer of the outer membrane. Chelating agents, such as ethylenediaminetetraacetic acid (EDTA), bind magnesium ions in the lipopolysaccharide layer and produce cells with increased susceptibility to antimicrobial molecules and detergents.17 When E coli or Bacteroides were exposed to cell wall–degrading enzymes such as lysozyme, no loss of optical density was observed; however, when they were combined with a chelator, a drop in density occurred rapidly. This is due to the fact that gram-negative bacteria have a protective outer membrane that is stabilized by metal ions, and a chelator is required to remove it (Figure 1).
- Enzymes. We selected lysozyme, which is found in tears and other secretions. It is responsible for breaking down the polysaccharide walls of many kinds of bacteria. Lysozyme is known to lyse gram-positive bacteria but has difficulty accessing the cell wall of gram-negative organisms.18 A chelator such as EDTA or citrate can be combined with lysozyme to effectively kill E coli and Bacteroides (Figure 1).
- Peptides. Nisin was selected as an antibacterial peptide that exhibits a broad spectrum of inhibitory activity against many colonic bacterial species. Kordel and Sahl17 showed that E coli exhibited nisin sensitivity.9,19-21 Nisin at 1 mg/mL could reduce and slow the growth of Bacteroides fragilis as seen in Figure 2.
- Essential oils. Essential oils include but are not limited to carvarcol; thymol; and oils of ginger, cinnamon, mint, onion, black cumin, oregano, thyme, clove, garlic, eucalyptus, lavender, leleshwa, lemon, lemon myrtle, neem, cilantro, tea tree, and peppermint. The composition, structure, and functional groups of the oils play an important role in determining their antimicrobial activity.22 Usually compounds with phenolic groups are most effective.9,12 Among these, the oils of clove, oregano, rosemary, thyme, sage, and vanillin have been found to be most consistently effective against microorganisms. Oregano, clove, cinnamon, and citral are effective against both groups of bacteria.23 Some nonphenolic constituents of oils, such as allyl isothiocyanate, are quite effective against gram-negative bacteria (Figure 3).
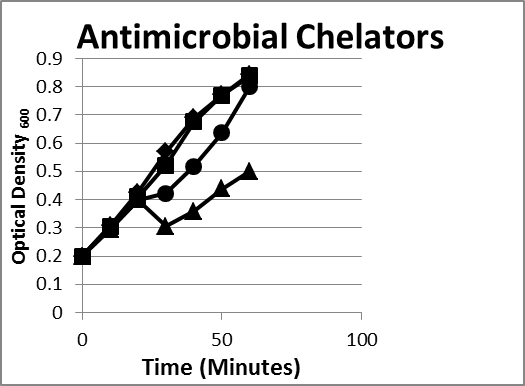
Figure 1. Inhibition/lysis profiles. (♦)Escherichia coli B, (■) lysozyme (1 mg/mL), (●) lactoferrin (1 mg/mL) + Ethylenediaminetetraacetic acid (EDTA) (1 mg/mL), (▲) EDTA+lysozyme (1 mg/mL). Growth curves of an enzyme and/or chelator reducing the growth of the E coli as evidenced by a reduction in the optical density reflecting lysis of some of the bacteria; however, in time the existing colonies began to multiply as evidenced by an increase in the optical density. Bacteroides species exhibited the same pattern.
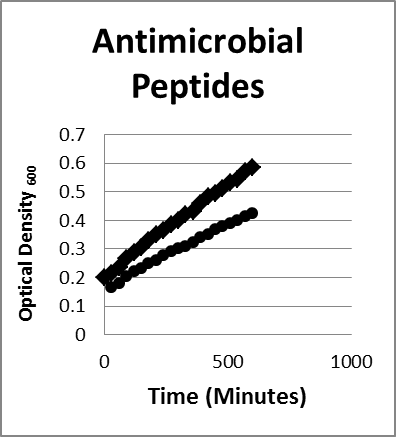
Figure 2. Inhibition/lysis profiles. (♦) Bacteroides fragilis control (●) Nisin growth curves of an a peptide reducing the growth of the B fragilis as evidenced by a reduction in the optical density reflecting lysis of some of the bacteria; Escherichia coli species exhibited the same growth and inhibition pattern under the same conditions.
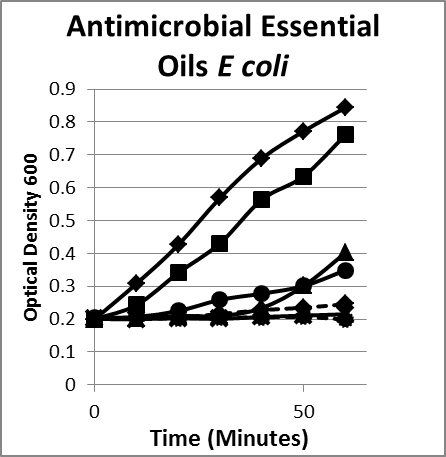
Figure 3A
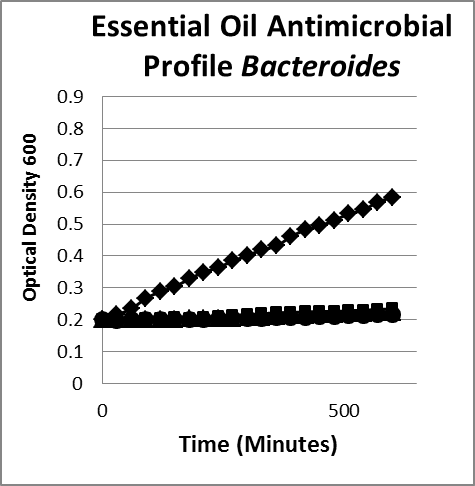
Figure 3B
Figure 3. Inhibition/lysis profiles. A. (♦) Escherichia coli B, (■) cinnamon extract (without cinnamaldehyde) (1 mg/mL), (●) clove oil (1 mg/mL), (✺) garlic (1 mg/mL), (X) oregano oil (1 mg/mL), (▲) peppermint leaf (1 mg/mL). Growth curves of essential oils that inhibited the growth of E coli as evidenced by a static optical density; however, the existing colonies begin to multiply as evidenced by an increase in the optical density after a period of time. B. (♦) Bacteroides fragilis, (■) oregano oil (1 mg/mL), (●) garlic (1 mg/mL), (▲) thyme (1 mg/mL). The suppression pattern of essential oils was the same with similar gut flora gram-negative bacteria Bacteroides (B) as it was with E coli (A), suggesting that the underlying mechanism and phenotype is the same for similar microorganisms.
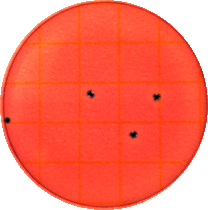
When exposed to antimicrobial formulation (Figure 4, plates B and C), the colony count is drastically reduced by 3 logs compared to the control. These results show that an antimicrobial formulation can adequately reduce and suppress growth of E coli under physiological conditions.


Figures 4A, 4B, and 4C.
Figure 4. Enumerations of Escherichia coli in the absence (plate A) and presence (plates B and C) of the antimicrobial formulation: the plates were diluted and plated at 1x103 after 6 hours of growth in nutrient media. E coli was reduced by more than 1000 colony counts for several hours.
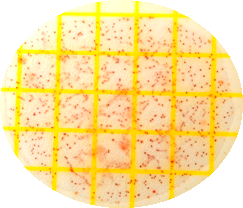
When exposed to antimicrobial formulation (Figure 4B), B subtilis growth is not affected when compared to the control (Figure 4A) in contrast with the marked reduction in suppression of the growth of E coli (comparing Figures 4 and 5). Side note: while the antimicrobial formulation does not kill B subtilis, it does inhibit the growth of the probiotic until the antimicrobial formula is diluted down to 0.01% of the original dilution. This dilution should also occur in the body, so it should not be an issue.

Figures 5A and 5B.
Figure 5. Enumerations of Bacillus subtilis in the absence (plate A) and presence (plate B) of the antimicrobial formulation 1, the plates were diluted and plated at 1x106 after 6 hours of growth in nutrient media. Unlike Escherichia coli, B subtilis was not reduced by the antimicrobial formulation at the same concentration.
Discussion
Bacteroides and E coli can be lysed using cell wall–degrading enzymes such as lysozyme as long as the outer membrane is compromised by something such as the chelator EDTA (Figure 1). Essential oils and peptides can also inhibit bacterial growth (Figures 2, 3, and 6) and are more effective when paired with an outer membrane destabilizer such as EDTA. The same phenotypes are observed when using the same antimicrobials on similar bacteria (Figures 3 and 6). A combination of these antimicrobials can reduce and suppress bacterial growth under small intestinal conditions (Figure 4). An actual count of the bacteria in the presence and absence of antimicrobial combination shows that a bacterial population can be reduced several logs even in the presence of solid foods (Figure 4) and inhibited for several hours. Spores, on the other hand, can survive the antimicrobial conditions losing very little vitality (Figure 5).
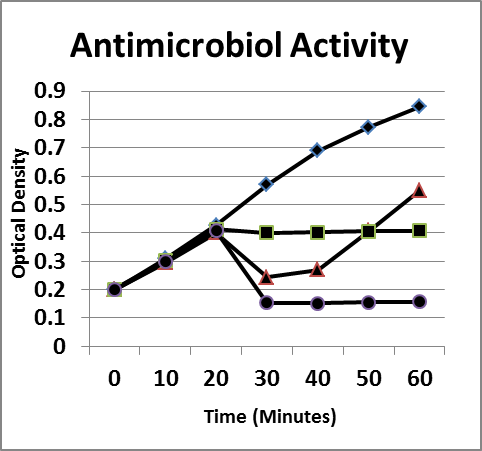
Figure 6. Inhibition/lysis profiles. A. (♦) Escherichia coli B, (■) oregano oil (1 mg/mL), (▲) lysozyme (1 mg/mL) + Ethylenediaminetetraacetic acid (1 mg/mL), (●) combination. Growth curves of essential oils that inhibited the growth of E coli as evidenced by a static optical density; however, the existing colonies begin to multiply as evidenced by an increase in the optical density after a period of time.
The clinical syndrome of SIBO is characterized by a quantitative and qualitative growth of microorganisms affecting the structure and function of the small intestine. Therapies are usually directed at reducing the numbers and inhibiting the growth of these microorganisms and supplanting them with beneficial bacteria such as Lactobacilli species.24 This study was aimed at measuring the ability of several natural ingredients to affect the growth of colonic microorganisms. The composition was selected from a group of enzymes, essential oils, chelators, and peptides. The probiotic B subtilis was included in the formula, and its growth was not affected by the associated antimicrobials. Teleologically, such blends may be of aid in the armamentarium for the management of patients with SIBOS in that there is a reduction and suppression of harmful bacterial growth while the accompanying probiotic recolonizes the intestines.
During small bacterial overgrowth in the small intestine, colonic bacteria such as Bacteroides and E coli contaminate the small intestine at 2 to 3 logs higher than the bacteria count normally present in healthy individuals. To promote healthy small intestinal conditions in people with SIBOS, colonic bacterial populations need to be reduced and inhibited to allow beneficial bacteria that normally reside in the small intestine to reestablish themselves and regain control of the intestine. Natural antimicrobials were screened at concentrations that could be formulated in a supplement to reduce or inhibit a variety of microorganisms. These concentrations were based on physiological conditions of 500 mL using a 1 mg/mL dosage, which would be an ideal formulation for this application. The recommended dosage was defined under physiological conditions and screened in order to reduce/lyse the bacterial population by 1 to 2 logs and inhibit microbial growth for 1 to 2 hours.
In vivo tests are required to determine whether the dosages used in vitro work in vivo. Delayed-release or enteric-coated capsules will contain this antimicrobial combination so it can be absorbed by the small intestine where it is needed. Each antimicrobial gave a unique profile in killing or inhibiting colonic bacteria growth in this research. Once the individual antimicrobial properties were obtained on the test bacteria, a combination was chosen of these natural antimicrobials that would function to both reduce and inhibit the colonic microbacteria under physiological conditions for a period of time to allow replenishment of good bacteria either present in the small intestine or provided in the formulation. B subtilis survives the antimicrobial formula and thus can be administered concomitantly. Other probiotics that can repopulate the small intestine may be used at additional intervals. Many of the above ingredients worked best with EDTA, suggesting that gram-negative bacteria require destabilization of the outer membrane in order for the antimicrobial to work against the bacteria.
These tests targeted 2 of the main bacterial contributors to SIBOS. It should be noted, however, that these tests were done in an isolated system in vitro under physiological conditions. Further research should include animal and human clinical trials, including tests to determine how these components interact with the inherent bacterial population present as well as the host itself.
Editor's note: This research study was funded in total by Deerland Enzymes, Inc, Kennesaw, Georgia. The study was designed to evaluate the efficacy of a dietary supplement manufactured and sold by Deerland Enzymes. No other sources of funding were used for this research study.










