______________________________________________________________
Cristiana Paul, MSa and David M. Brady, ND, DACBN, IFMCP, FACNb
A recent study by Witkowski M., et al., has investigated correlations between various plasma “metabolomics,” including sugar alcohols, such as erythritol (ET), and cardiovascular disease (CVD) events.1 Plasma ET may be derived from either endogenous production of ET (glucose or fructose) or exogenous intake of ET, some naturally occurring in fruits and some as a food sweeteners. Data included two cohorts, one in the U.S. (n = 2,149) and one in Europe (n = 833), and both were followed for three years; however, it did not include the intake of dietary ET or other carbohydrates, which is a severe limitation of the cohorts’ analyses.1 Fasting blood ET levels were assessed only once at enrollment in
The correlation analyses found that the highest quartile of blood ET levels had significantly more CVD events than the lowest quartile of blood ET levels.1 It is well known that correlations do not always imply causation, as also stated by the authors. Most importantly, their analysis cannot be used to imply that the intake of ET raises CVD risk because ET intake was not assessed.1 Various news outlets picked up the wording from the correlation results and spun this into the headline, “Erythritol sweetener may increase
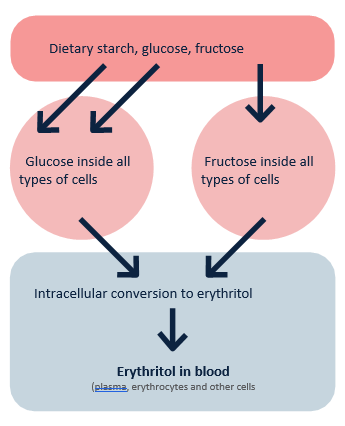
Fig. 1. Endogenous conversion of glucose and fructose into erythritol. Adapted from Witkowski M., et al. Mammalian metabolism of erythritol: a predictive biomarker of metabolic dysfunction.
The causal effect between glucose and/or fructose and CVD is well established (e.g., its impacts on cholesterol, triglycerides, insulin, blood pressure, and thrombosis). Thus, it is very likely that the correlation between high blood levels of ET and CVD may be due, at least in part, to the vascular and lipid problems caused by an excess intake of glucose, fructose, and/or starch.
A 2023 study from the journal Nutrients addressed the issue in question and noted “observational studies indicate positive association between plasma erythritol and obesity and cardiometabolic disease.”3 The authors’ explanation of this phenomena suggests that elevated blood ET is a mere marker of excess intake of caloric sugars and sugar-specific effects as the underlying culprits “It is unlikely that dietary erythritol is mediating these associations, rather they reflect dysregulated pentose phosphate pathway due to impaired glycemia or glucose-rich diet.”3
The 2023 study by Witkowski M., et al., also included an in vitro experiment that showed adding ET to platelet cells increased platelet aggregation.1 However, adding ET to a medium with platelets does not necessarily reproduce the effects of ingesting ET and how it is metabolized or incorporated as such in various body and blood cells or plasma. They showed a similar effect in an animal model but also
The same study by Witkowski M. et al. included a small (n = 8) intervention with eight subjects that were given 30 g of ET and measured plasma ET for 7 days. They found the resulting ET plasma levels to
Another 2023 study and a 2018 study reviewed many potential benefits of ET, including its following properties and functions: as an antioxidant, promoting flow-mediated dilation, non-insulinemic, non- glycemic, non-caloric (with the majority being excreted in the urine unmetabolized), no impact on blood lipids, no gastrointestinal distress, and beneficial effects on oral health (demonstrated by reduced plaque and pathogenic oral bacteria). The authors of both studies ultimately recommended the use of ET as a beneficial substitute for glucose or fructose.3,4
According to the Food and Drug Administration (written communication, June 5, 2018), “…erythritol, described in the enclosed notification is exempt from the premarket approval requirement of the Federal Food, Drug, and Cosmetic Act because it has been determined to be generally recognized as safe (GRAS), based on scientific procedures, for addition to food.” Erythritol is a naturally occurring compound found in a variety of foods and beverages, including fruits (e.g., melons, pears, and grapes) at levels of 10-20 mg/lb, soy sauce at 0.9 mg/L, wine at 300 mg/L, miso paste at 1.3 mg/L, and sake at 1.5 mg/L.5 Consumption of a few common servings from a variety of such foods may add up to less than 0.5 g/day.






